EGFL7 Inhibits the Sestrin2/Nrf2 Signaling Pathway,promotes Angiogenesis,and aggravates Diabetic Retinopathy in Rats
-
摘要:
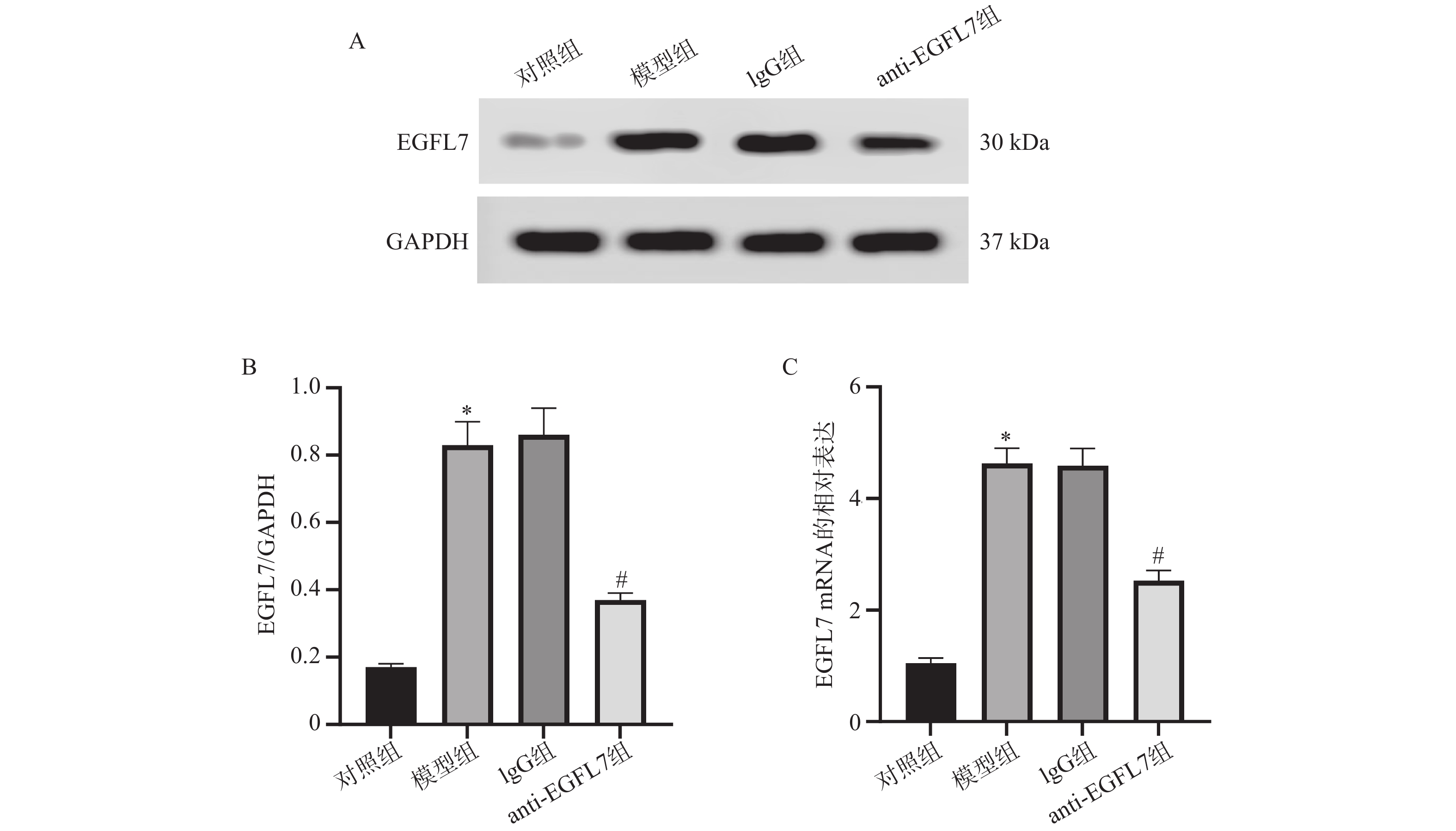
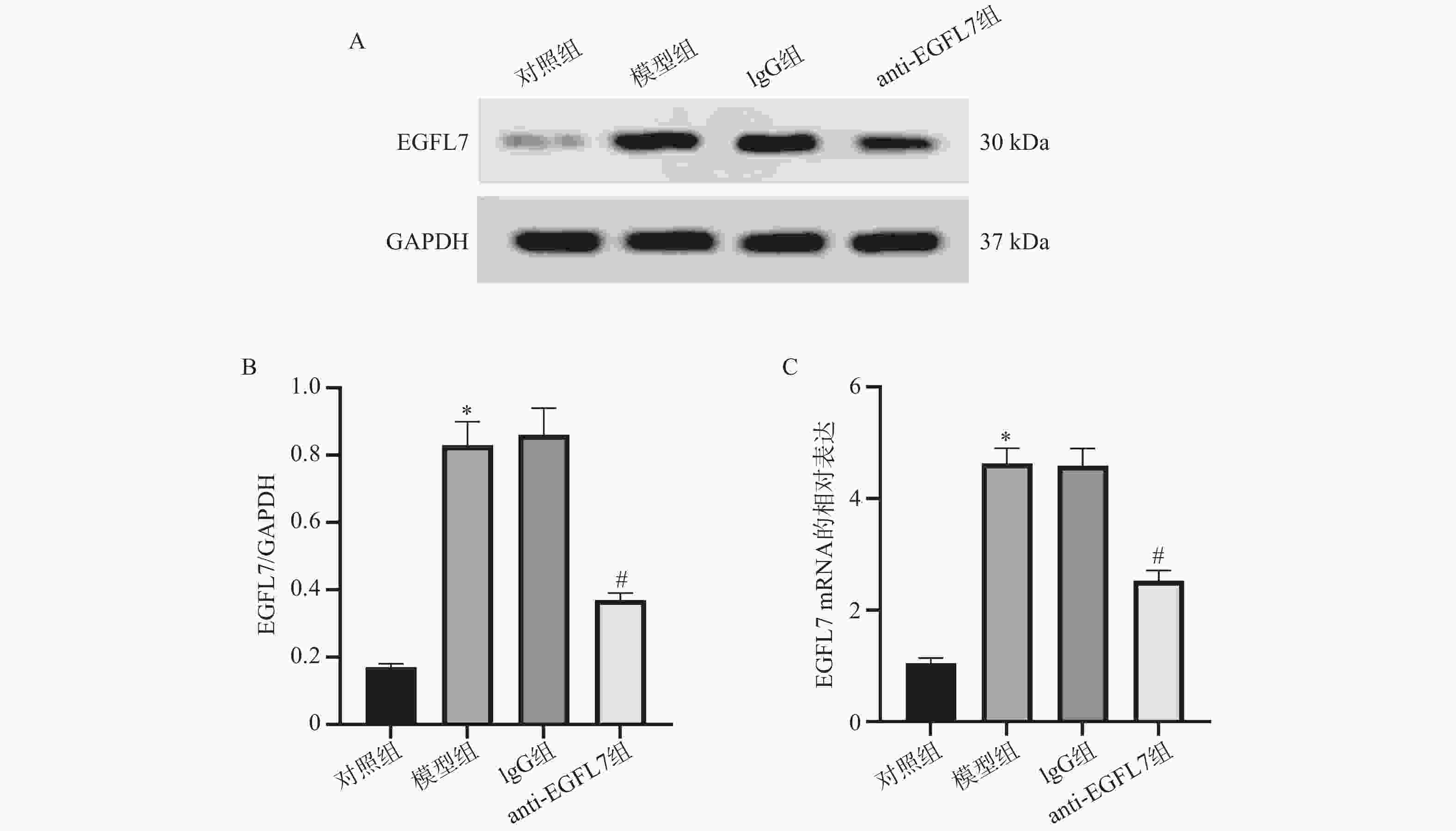
目的 基于Sestrin2/核因子红细胞2相关因子2(nuclear factor erythroid 2-related factor 2,Nrf2)信号通路,探究表皮生长因子样结构域7(epidermal growth factor-like domain 7,EGFL7)对糖尿病视网膜病变血管生成的影响。 方法 用大鼠构建糖尿病视网膜病变模型。苏木精-伊红(hematoxylin-eosin,HE)染色用于观察视网膜病理学形态。视网膜胰蛋白酶消化实验用于检测视网膜血管形态。实时定量聚合酶链式反应(real-time quantitative polymerase chain reaction,RT-qPCR)和蛋白免疫印迹(Western blot)分别用于分析视网膜组织EGFL7、Sestrin2、Nrf2、血红素氧合酶1(heme oxygenase-1,HO-1)的mRNA表达以及EGFL7、Sestrin2、分化簇31(cluster of differentiation 31,CD31)、血管内皮生长因子受体2(vascular endothelial growth factor receptor 2,VEGFR2)、Nrf2、HO-1的蛋白表达。相应试剂盒用于检测眼内房水中丙二醛(malondialdehyde,MDA)、超氧化物歧化酶(superoxide dismutase,SOD)、谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-Px)的水平。 结果 与对照组相比,模型组大鼠的视网膜组织呈现病理学损伤,视网膜组织中血管数量增加,EGFL7、CD31、VEGFR2、MDA升高(P < 0.05),SOD、GSH-Px、Sestrin2、Nrf2、HO-1降低(P < 0.05);与模型组相比,IgG组大鼠的上述病理学指标无显著变化(P > 0.05);与IgG组相比,anti-EGFL7组大鼠视网膜组织的病理学损伤减轻,视网膜组织中血管数量减少,EGFL7、CD31、VEGFR2、MDA降低(P < 0.05),SOD、GSH-Px、Sestrin2、Nrf2、HO-1升高(P < 0.05)。 结论 EGFL7可能通过促进氧化应激来加剧糖尿病视网膜病变大鼠视网膜的血管新生,其机制可能与抑制Sestrin2/Nrf2信号通路相关。 -
关键词:
- EGFL7 /
- 糖尿病视网膜病变 /
- Sestrin2/Nrf2 /
- 氧化应激
Abstract:Objective To investigate the impact of epidermal growth factor-like domain 7 (EGFL7) on angiogenesis in diabetic retinopathy, based on the Sestrin2/nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway. Methods A diabetic retinopathy model was established in the rats. Retinal pathological morphology was examined using hematoxylin-eosin (HE) staining, while retinal vascular morphology was assessed via the retinal trypsin digestion assay. The mRNA expression levels of EGFL7, Sestrin2, Nrf2, and heme oxygenase-1 (HO-1) in retinal tissues were analyzed by RT-qPCR. Protein expression levels of EGFL7, Sestrin2, cluster of differentiation 31 (CD31), vascular endothelial growth factor receptor 2 (VEGFR2), Nrf2, and HO-1 were detected by Western blot. Corresponding assay kits were used to measure the levels of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) in the intraocular aqueous humor. Results Compared with the control group, rats in the model group exhibited pathological damage in retinal tissues, increased retinal vascular density, and elevated levels of EGFL7, CD31, VEGFR2, and MDA (P < 0.05), whereas levels of SOD, GSH-Px, Sestrin2, Nrf2, and HO-1 were decreased (P < 0.05). No significant changes were observed in the above pathological indicators in the IgG group compared with the model group (P > 0.05). In contrast, compared with the IgG group, rats in the anti-EGFL7 group showed alleviated retinal pathological injury, reduced retinal vascular density, decreased levels of EGFL7, CD31, VEGFR2, and MDA (P < 0.05), and increased levels of SOD, GSH-Px, Sestrin2, Nrf2, and HO-1 (P < 0.05). Conclusion EGFL7 may exacerbate retinal neovascularization in diabetic retinopathy rats by promoting oxidative stress, a mechanism potentially associated with the inhibition of the Sestrin2/Nrf2 signaling pathway. -
Key words:
- EGFL7 /
- Diabetic retinopathy /
- Sestrin2/Nrf2 /
- Oxidative stress
-
图 7 Western blot实验检测各组大鼠视网膜中EGFL7分子及Sestrin2/Nrf2信号通路相关分子的表达($ \bar x \pm s $,n = 8)
A:Western blot条带图;B:Sestrin2/GAPDH;C:Nrf2(总)/GAPDH;D:HO-1/GAPDH;E:Nrf2(胞浆)/GAPDH;F:Nrf2(胞核)/Histone;与对照组相比,*P < 0.05;与IgG组相比,#P < 0.05。
Figure 7. Western blot experiment detected the expression of EGFL7 molecules and Sestrin2/Nrf2 signaling pathway-related molecules in the retina of rats in each group ($ \bar x \pm s $,n = 8)
表 1 引物信息表
Table 1. Primer information table
引物名称 引物序列 引物长度(bp) EGFL7-F 5'- TGCTGATGTGGCTTCTGGTGTTG -3' 21 EGFL7-R 5'- GGTGGTGAGGAAGGGCTGGTAC -3' 22 Sestrin2-F 5'- CTTCATCCCAGTGGAGGAGATCC -3' 23 Sestrin2-R 5'- CCAGAAGCTGCTAAGGTAGTCG -3' 21 Nrf2-F 5'- CCCATTGAGGGCTGTGATCT -3' 20 Nrf2-R 5'- GCCTTCAGTGTGCTTCTGGTT -3' 20 HO-1-F 5'- ATTTGTCCGAGGCCTTGAA -3' 19 HO-1-R 5'- CCAGGGCCGTATAGATATGGTA -3' 22 GAPDH-F 5'- ACAGCAACAGGGTGGTGGAC -3' 20 GAPDH-R 5'- TTTGAGGGTGCAGCGAACTT -3' 20 -
[1] Teo Z L, Tham Y C, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis[J]. Ophthalmology, 2021, 128(11): 1580-1591. doi: 10.1016/j.ophtha.2021.04.027 [2] Chang W, Lajko M, Fawzi A A. Endothelin-1 is associated with fibrosis in proliferative diabetic retinopathy membranes[J]. PLoS One, 2018, 13(1): e0191285. doi: 10.1371/journal.pone.0191285 [3] Katari V, Dalal K, Adapala R K, et al. A TRP to pathological angiogenesis and vascular normalization[J]. Compr Physiol, 2024, 14(2): 5389-5406. doi: 10.1002/j.2040-4603.2024.tb00295.x [4] Nichol D, Stuhlmann H. EGFL7: A unique angiogenic signaling factor in vascular development and disease[J]. Blood, 2012, 119(6): 1345-1352. doi: 10.1182/blood-2011-10-322446 [5] Xian Y, Wang X, Yu Y, et al. The mechanism of EGFL7 regulating neovascularization in diabetic retinopathy through the PI3K/AKT/VEGFA pathway[J]. Life Sci, 2024, 340: 122483. doi: 10.1016/j.lfs.2024.122483 [6] Du X, Wang Y, Gao F. PSAT1 is upregulated by METTL3 to attenuate high glucose-induced retinal pigment epithelial cell apoptosis and oxidative stress[J]. Diagn Pathol, 2024, 19(1): 138. doi: 10.1186/s13000-024-01556-4 [7] Xi X, Chen Q, Ma J, et al. Sestrin2 ameliorates diabetic retinopathy by regulating autophagy and ferroptosis[J]. J Mol Histol, 2024, 55(2): 169-184. doi: 10.1007/s10735-023-10180-3 [8] Kishimoto Y, Kondo K, Momiyama Y. The protective role of Sestrin2 in atherosclerotic and cardiac diseases[J]. Int J Mol Sci, 2021, 22(3): 1200. doi: 10.3390/ijms22031200 [9] Pasha M, Eid A H, Eid A A, et al. Sestrin2 as a novel biomarker and therapeutic target for various diseases[J]. Oxid Med Cell Longev, 2017, 2017: 3296294. doi: 10.1155/2017/3296294 [10] Yang X, Li D. Tricin attenuates diabetic retinopathy by inhibiting oxidative stress and angiogenesis through regulating Sestrin2/Nrf2 signaling[J]. Hum Exp Toxicol, 2023, 42: 9603271231171642. [11] 帅天姣, 王彤彤, 谢伟, 等. 黄芩苷调节IL-33/ST2信号通路对糖尿病视网膜病变大鼠视网膜新生血管生成的影响[J]. 眼科新进展, 2022, 42(9): 685-689. [12] Bill M, Pathmanathan A, Karunasiri M, et al. EGFL7 antagonizes NOTCH signaling and represents a novel therapeutic target in acute myeloid leukemia[J]. Clin Cancer Res, 2020, 26(3): 669-678. doi: 10.1158/1078-0432.CCR-19-2479 [13] Liu G, Feng L, Liu X, et al. O-GlcNAcylation inhibition upregulates Connexin43 expression in the endothelium to protect the tight junction barrier in diabetic retinopathy[J]. Invest Ophthalmol Vis Sci, 2023, 64(14): 30. doi: 10.1167/iovs.64.14.30 [14] Hu A, Schmidt M H H, Heinig N. Microglia in retinal angiogenesis and diabetic retinopathy[J]. Angiogenesis, 2024, 27(3): 311-331. doi: 10.1007/s10456-024-09911-1 [15] Dănilă A I, Ghenciu L A, Stoicescu E R, et al. Aldose reductase as a key target in the prevention and treatment of diabetic retinopathy: A comprehensive review[J]. Biomedicines, 2024, 12(4): 747. doi: 10.3390/biomedicines12040747 [16] Nichol D, Shawber C, Fitch M J, et al. Impaired angiogenesis and altered Notch signaling in mice overexpressing endothelial Egfl7[J]. Blood, 2010, 116(26): 6133-6143. doi: 10.1182/blood-2010-03-274860 [17] Badiwala M V, Guha D, Tumiati L, et al. Epidermal growth factor-like domain 7 is a novel inhibitor of neutrophil adhesion to coronary artery endothelial cells injured by calcineurin inhibition[J]. Circulation, 2011, 124(11 Suppl): S197-S203. [18] Carvalho M I, Silva-Carvalho R, Prada J, et al. TGFβ in malignant canine mammary tumors: Relation with angiogenesis, immunologic markers and prognostic role[J]. Vet Q, 2024, 44(1): 1-12. [19] Yu E, Kim H, Park H, et al. Targeting the VEGFR2 signaling pathway for angiogenesis and fibrosis regulation in neovascular age-related macular degeneration[J]. Sci Rep, 2024, 14(1): 25682. doi: 10.1038/s41598-024-76258-4 [20] Peng J, Abdulla R, Liu X, et al. Polyphenol-rich extract of Apocynum venetum L. leaves protects human retinal pigment epithelial cells against high glucose-induced damage through polyol pathway and autophagy[J]. Nutrients, 2024, 16(17): 2944. doi: 10.3390/nu16172944 [21] Xu Y, Yu J. Allicin mitigates diabetic retinopathy in rats by activating phosphatase and tensin homolog-induced kinase 1/parkin-mitophagy and inhibiting oxidative stress-mediated NOD-like receptor family pyrin domain containing 3 inflammasome[J]. J Physiol Investig, 2024, 67(4): 215-224. doi: 10.4103/ejpi.EJPI-D-24-00039 [22] Hou Z, Wang Z, Zhang J, et al. Effects of cannabidiol on AMPKα2/HIF-1α/BNIP3/NIX signaling pathway in skeletal muscle injury[J]. Front Pharmacol, 2024, 15: 1450513. doi: 10.3389/fphar.2024.1450513 [23] Datta S, Ghosh S, Bishayee A, et al. Flexion of Nrf2 by tea phytochemicals: A review on the chemopreventive and chemotherapeutic implications[J]. Pharmacol Res, 2022, 182: 106319. doi: 10.1016/j.phrs.2022.106319 [24] Nakamura S, Noguchi T, Inoue Y, et al. Nrf2 activator RS9 suppresses pathological ocular angiogenesis and hyperpermeability[J]. Invest Ophthalmol Vis Sci, 2019, 60(6): 1943-1952. doi: 10.1167/iovs.18-25745 -





 下载:
下载: