Mechanism of HDAC3-targeted Regulation of GATA-2 Affecting Osteogenic Differentiation of Dental Pulp Stem Cells
-
摘要:
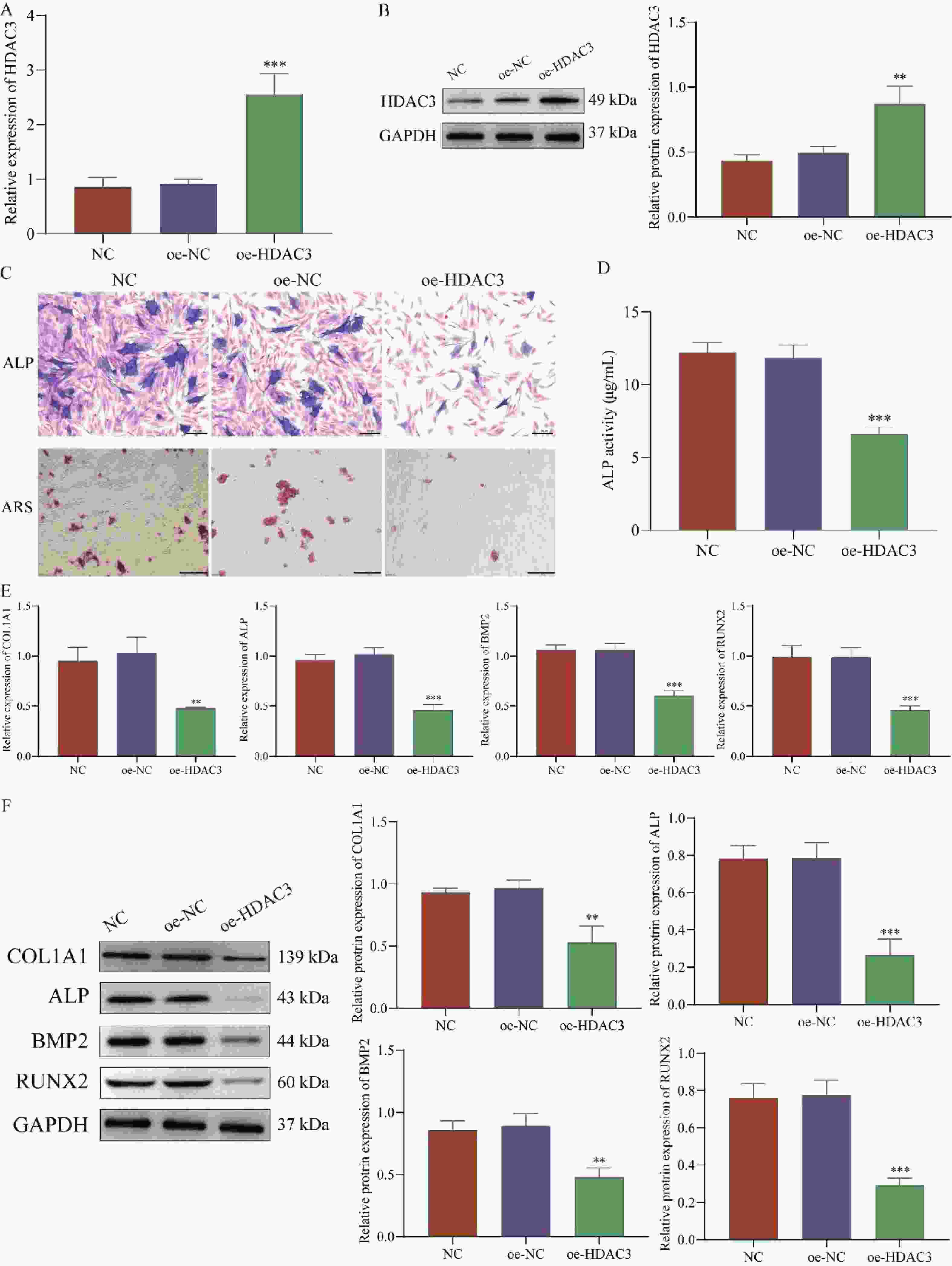
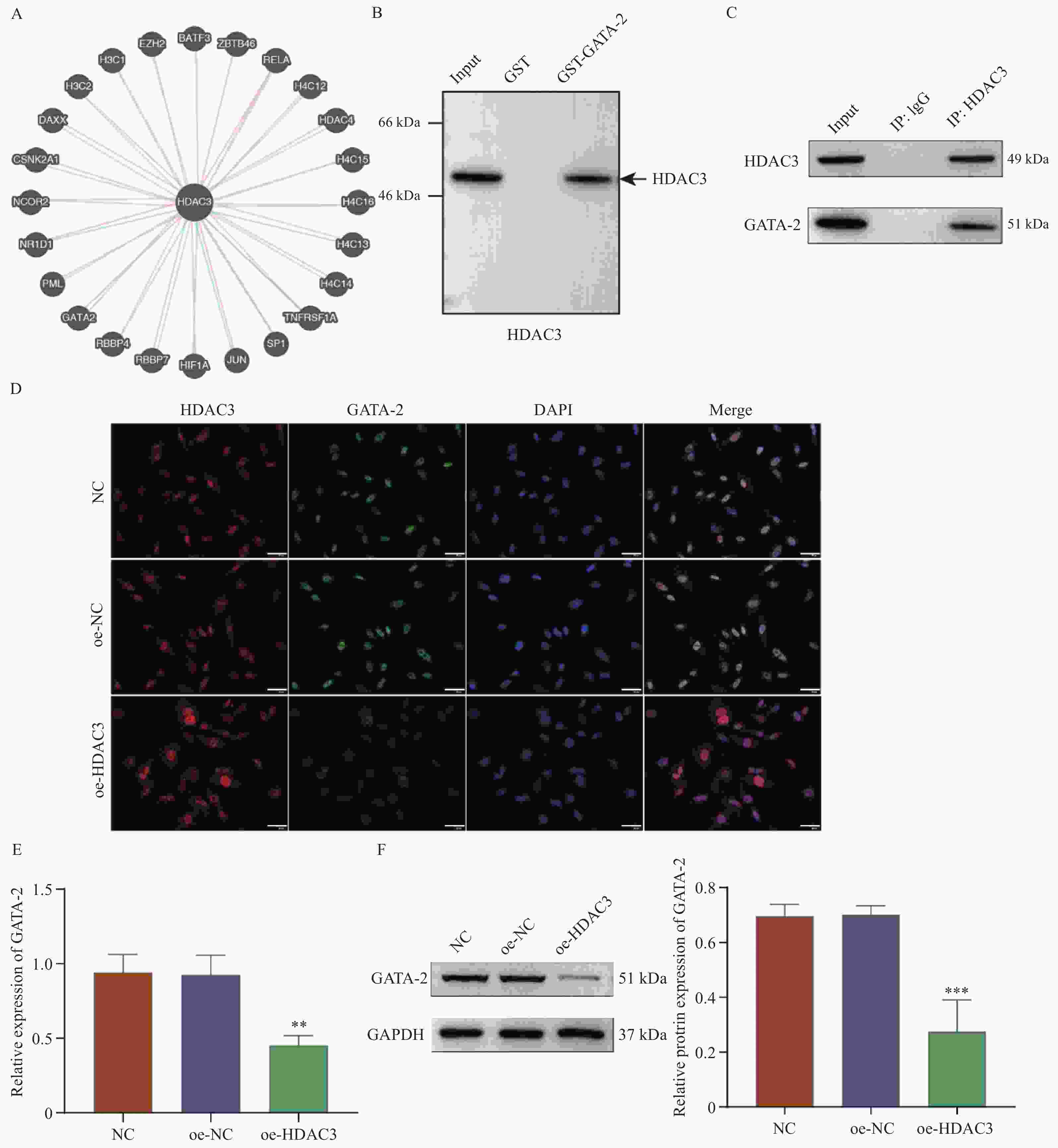
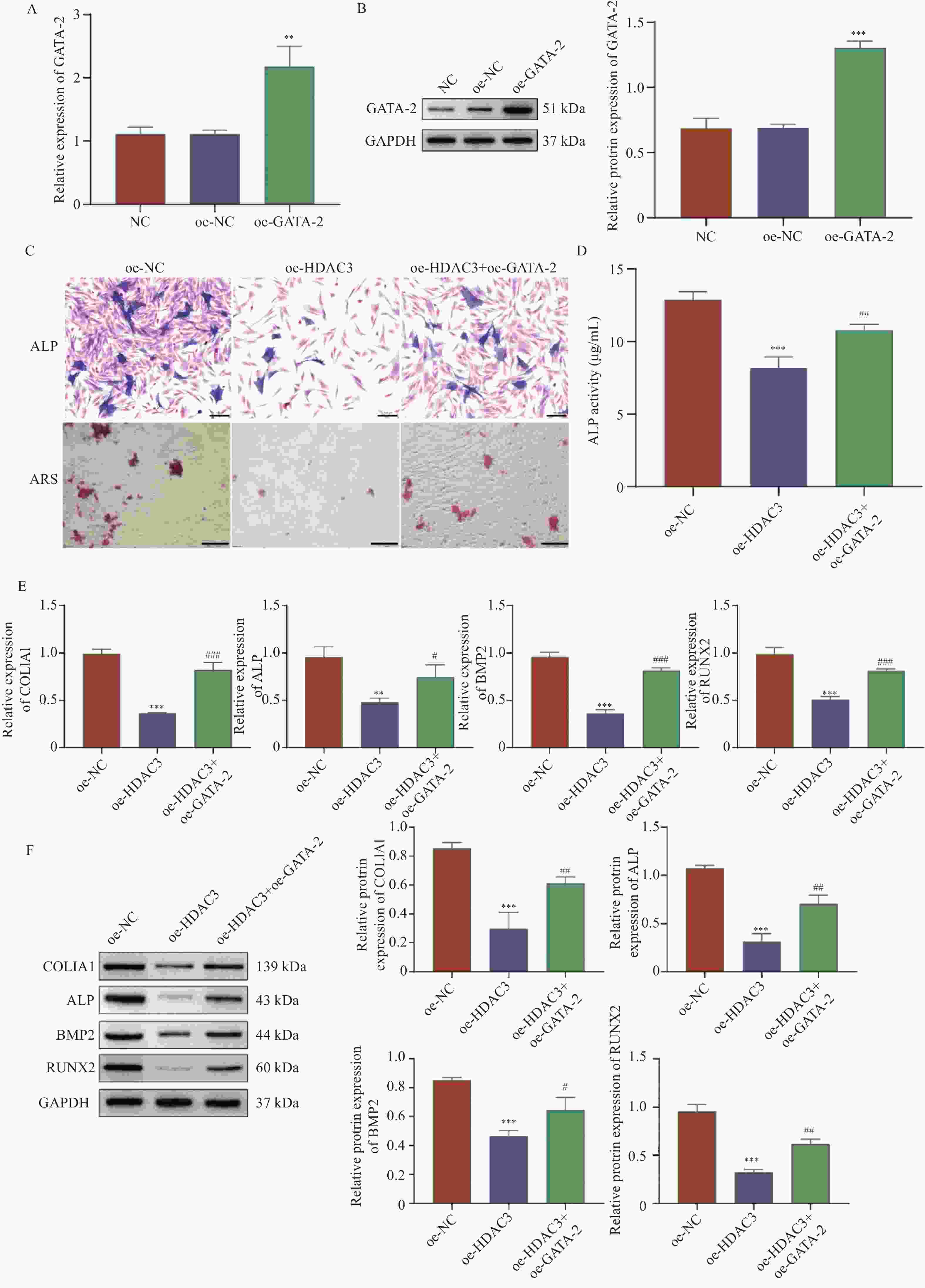
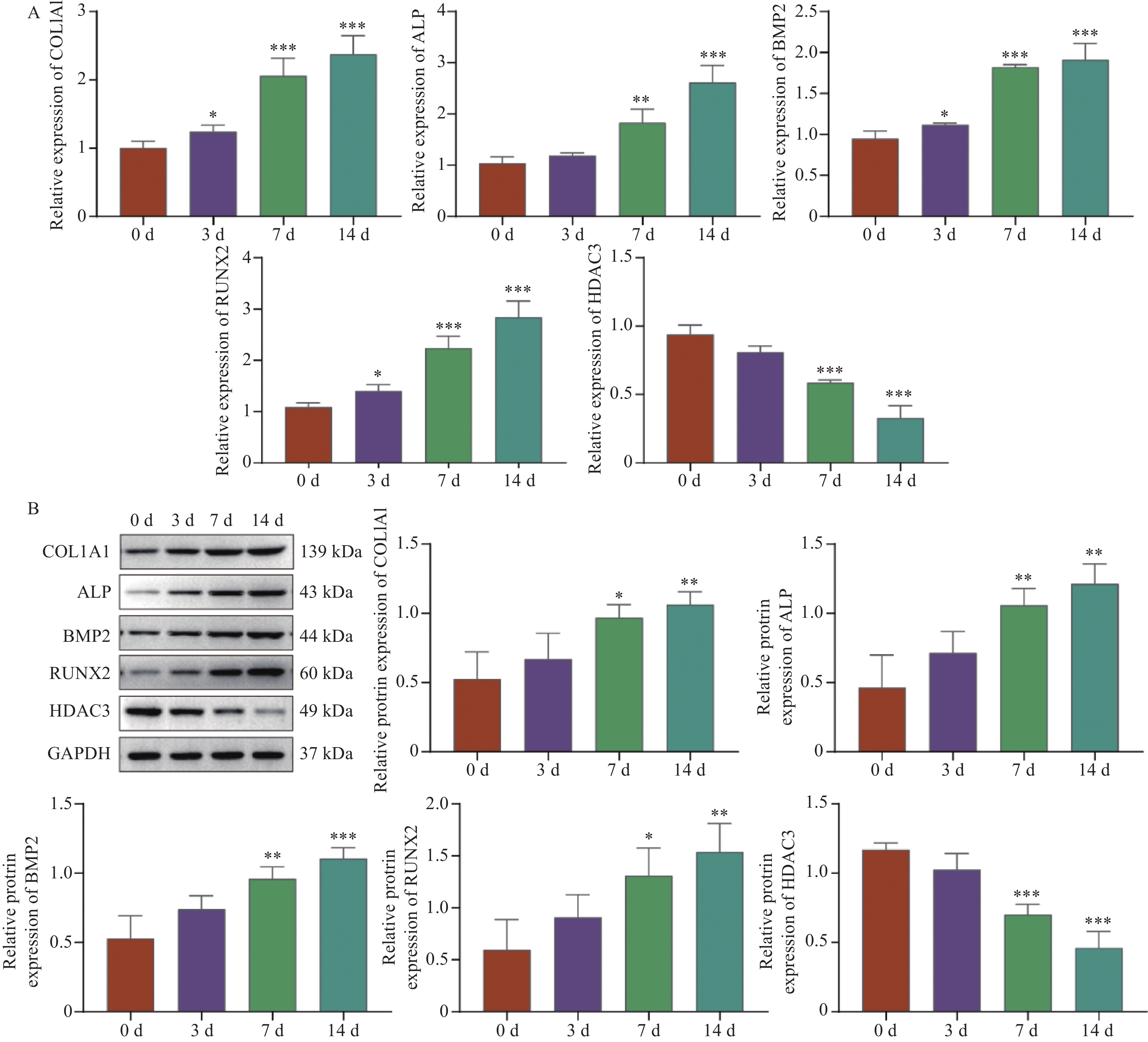
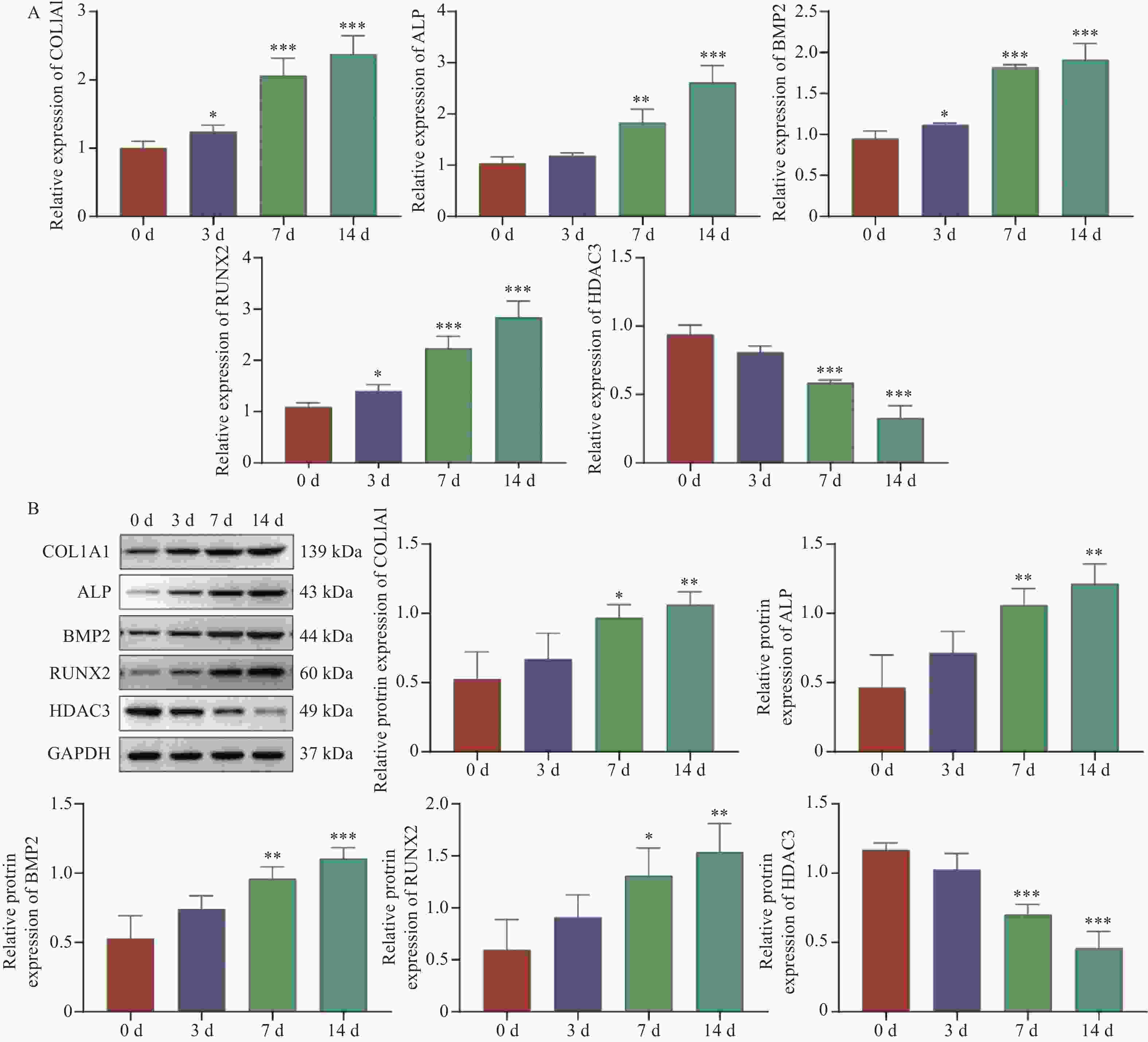
目的 探讨HDAC3/GATA-2分子轴在人牙髓干细胞(human dental pulp stem cells,hDPSCs)成骨分化中的作用机制。 方法 hDPSCs细胞系通过骨诱导培养基(osteogenic induction medium,OM)培养,构建oe-NC,oe-HDAC3和oe-GATA-2质粒,并转染至细胞中,转染效率通过RT-qPCR和Western blot进行评估。采用碱性磷酸酶(alkaline phosphatase,ALP)染色和茜素红S(alizarin red S,ARS)染色评估ALP活性以及细胞的矿化节点。RT-qPCR和Western Blot检测成骨关键指标COL1A1、ALP、BMP2和RUNX2的表达水平。GST-pull down和免疫共沉淀(co-immunoprecipitation,CO-IP)验证HDAC3与GATA-2的相互作用,免疫荧光(immunofluorescence,IF)染色检测细胞表达。 结果 HDAC3在hDPSCs成骨分化过程中显著降低(P < 0.0001 ),过表达HDAC3显著减弱ALP染色和活性(P =0.0001 ),减少hDPSCs细胞矿化结节的产生。COL1A1(P =0.0029 )、ALP(P =0.0001 )、BMP2(P =0.0001 )和RUNX2(P =0.0007 )的表达水平在过表达HDAC3后被抑制。HDAC3与GATA-2相互作用,与过表达HDAC3降低了细胞中GATA-2的表达(P =0.0028 )。共转染oe-GATA-2后逆转了过表达HDAC3的作用,增加了ALP染色和活性(P =0.0043 )和细胞中的矿化结节。oe-HDAC3+ oe-GATA-2组中COL1A1(P =0.0001 )、ALP(P =0.0423 )、BMP2(P <0.0001 )和RUNX2(P =0.0005 )的表达水平进一步升高。结论 HDAC3通过靶向负调控GATA-2的表达,抑制了hDPSCs的成骨分化。 Abstract:Objective To investigate the mechanism of the HDAC3/GATA-2 molecular axis in osteogenic differentiation of human dental pulp stem cells (hDPSCs). Methods The hDPSCs cell line was cultured in osteogenic induction medium (OM). Plasmids of oe-NC, oe-HDAC3 and oe-GATA-2 were constructed and transfected into the cells. Transfection efficiency was assessed by RT-qPCR and Western blot. Alkaline phosphatase (ALP) staining and alizarin red S (ARS) staining were used to evaluate ALP activity and mineralization nodules in cells. RT-qPCR and Western Blot were used to detect the expression levels of osteogenic key indicators COL1A1, ALP, BMP2, and RUNX2. GST-pull down and Co-Immunoprecipitation (CO-IP) were used to validate the interaction of HDAC3 and GATA-2 interaction, and immunofluorescence (IF) staining was performed to detect cellular expression. Results HDAC3 expression was significantly reduced during osteogenic differentiation of hDPSCs (P < 0.0001 ). Overexpression of HDAC3 significantly attenuated ALP staining and activity (P =0.0001 ), as well as reduced the production of mineralized nodules in hDPSCs cells. The expression levels of COL1A1 (P =0.0029 ), ALP (P =0.0001 ), BMP2 (P =0.0001 ) and RUNX2 (P =0.0007 ) were inhibited after overexpression of HDAC3. HDAC3 interacted with GATA-2, and overexpression of HDAC3 decreased the expression of GATA-2 in cells (P =0.0028 ). Co-transfection of oe-GATA-2 reversed the inhibitory effects of HDAC3 overexpression, increasing ALP staining and activity (P =0.0043 ) and mineralization nodules in cells. In the oe-HDAC3 + oe-GATA-2 group, the expression levels of COL1A1 (P =0.0001 ), ALP (P =0.0423 ), BMP2 (P <0.0001 ), and RUNX2 (P =0.0005 ) were further elevated.Conclusion HDAC3 inhibited the osteogenic differentiation of hDPSCs by targeting and negatively regulating the expression of GATA-2. -
Key words:
- Human dental pulp stem cells /
- Osteogenic differentiation /
- HDAC3 /
- GATA-2
-
图 4 HDAC3通过下调GATA-2抑制hDPSCs成骨分化
A:RT-qPCR;B:Western blot检测转染效率;C:ALP染色(Scale bar=100 μm)和ARS染色(Scale bar=200 μm);D:试剂盒检测ALP活性;E:RT-qPCR;F:Western blot检测COL1A1、ALP、BMP2和RUNX2的mRNA和蛋白表达;与oe-NC组相比,*P < 0.05,**P < 0.01,***P < 0.001;与oe-HDAC3组相比,#P < 0.05;##P < 0.01;###P < 0.001。
Figure 4. HDAC3 inhibits hDPSCs osteogenic differentiation through downregulation of GATA-2
表 1 PCR引物序列
Table 1. PCR primer sequences
基因 引物 序列(5'-3') HDAC3 正向 5'-GGTGGTTATACTGTCCGAAAT-3' 反向 5'-AGGCTGAAGTCCCTGCTC-3' COL1A1 正向 5'-AAGGTGTTGTGCGATGACG-3' 反向 5'-ACCACGAGGACCAGAGGGAC-3' ALP 正向 5'-GGCTGGACGGGAAGAATC-3' 反向 5'-GCCTCCGAAGGAGAAGACG-3' BMP2 正向 5'-AACCTGCAACAGCCAACT-3' 反向 5'-GGGAGCCACAATCCAGTC-3' RUNX2 正向 5'-TGGGCTTCCTGCCATCAC-3' 反向 5'-CAGCGTCAACACCATCATTC-3' GATA-2 正向 5'-CTCCTGACCCTAGCACCACG-3' 反向 5'-GGTTCTGCCCATTCATCTTGT-3' GAPDH 正向 5'-TGACCACAGTCCATGCCATCAC-3' 反向 5'-CGCCTGCTTCACCACCTTCTT-3' -
[1] Han Y, You X, Xing W, et al. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts[J]. Bone Res, 2018, 6: 16. doi: 10.1038/s41413-018-0019-6 [2] Fernandes G, Yang S. Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering[J]. Bone Res, 2016, 4: 16036. doi: 10.1038/boneres.2016.36 [3] Ambrosi T H, Scialdone A, Graja A, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration[J]. Cell Stem Cell, 2017, 20(6): 771-784. e6. [4] Chen X Y, Xu S Z, Wang X W, et al. Systematic comparison of biologically active foreign ions-codoped calcium phosphate microparticles on osteogenic differentiation in rat osteoporotic and normal mesenchymal stem cells[J]. Oncotarget, 2017, 8(22): 36578-36590. doi: 10.18632/oncotarget.16618 [5] Camargo W A, de Vries R, van Luijk J, et al. Diabetes mellitus and bone regeneration: A systematic review and meta-analysis of animal studies[J]. Tissue Eng Part B Rev, 2017, 23(5): 471-479. doi: 10.1089/ten.teb.2016.0370 [6] Yamada Y, Ito K, Nakamura S, et al. Promising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrow[J]. Cell Transplant, 2011, 20(7): 1003-1013. doi: 10.3727/096368910X539128 [7] Ledesma-Martínez E, Mendoza-Núñez V M, Santiago-Osorio E. Mesenchymal stem cells derived from dental pulp: A review[J]. Stem Cells Int, 2016, 2016(1): 1-12. doi: 10.1155/2016/4709572 [8] Fu X, Jin L, Ma P, et al. Allogeneic stem cells from deciduous teeth in treatment for periodontitis in miniature swine[J]. J Periodontol, 2014, 85(6): 845-851. doi: 10.1902/jop.2013.130254 [9] Ge X, Li Z, Jing S, et al. Parathyroid hormone enhances the osteo/odontogenic differentiation of dental pulp stem cells via ERK and P38 MAPK pathways[J]. J Cell Physiol, 2020, 235(2): 1209-1221. doi: 10.1002/jcp.29034 [10] Edderkaoui M, Xu S, Chheda C, et al. HDAC3 mediates smoking-induced pancreatic cancer[J]. Oncotarget, 2016, 7(7): 7747-7760. doi: 10.18632/oncotarget.6820 [11] Cantley M D, Bartold P M, Marino V, et al. Histone deacetylase inhibitors and periodontal bone loss[J]. J Periodontal Res, 2011, 46(6): 697-703. doi: 10.1111/j.1600-0765.2011.01392.x [12] Schroeder T M, Kahler R A, Li X, et al. Histone deacetylase 3 interacts with runx2 to repress the osteocalcin promoter and regulate osteoblast differentiation[J]. J Biol Chem, 2004, 279(40): 41998-42007. doi: 10.1074/jbc.M403702200 [13] Ling K W, Ottersbach K, van Hamburg J P, et al. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells[J]. J Exp Med, 2004, 200(7): 871-882. doi: 10.1084/jem.20031556 [14] Rodrigues N P, Tipping A J, Wang Z, et al. GATA-2 mediated regulation of normal hematopoietic stem/progenitor cell function, myelodysplasia and myeloid leukemia[J]. Int J Biochem Cell Biol, 2012, 44(3): 457-460. doi: 10.1016/j.biocel.2011.12.004 [15] Kamata M, Okitsu Y, Fujiwara T, et al. GATA2 regulates differentiation of bone marrow-derived mesenchymal stem cells[J]. Haematologica, 2014, 99(11): 1686-1696. doi: 10.3324/haematol.2014.105692 [16] Lee E C, Kim Y M, Lim H M, et al. The histone deacetylase inhibitor (MS-275) promotes differentiation of human dental pulp stem cells into odontoblast-like cells independent of the MAPK signaling system[J]. Int J Mol Sci, 2020, 21(16): 57-71. [17] 任小华. LncRNA LOC101928855调控炎性环境下牙髓干细胞成骨分化的机制研究[D]. 成都: 电子科技大学, 2022. [18] Cakouros D, Gronthos S. Epigenetic regulation of bone marrow stem cell aging: Revealing epigenetic signatures associated with hematopoietic and mesenchymal stem cell aging[J]. Aging Dis, 2019, 10(1): 174-189. doi: 10.14336/AD.2017.1213 [19] Xu S, De Veirman K, Evans H, et al. Effect of the HDAC inhibitor vorinostat on the osteogenic differentiation of mesenchymal stem cells in vitro and bone formation in vivo[J]. Acta Pharmacol Sin, 2013, 34(5): 699-709. doi: 10.1038/aps.2012.182 [20] Paino F, La Noce M, Tirino V, et al. Histone deacetylase inhibition with valproic acid downregulates osteocalcin gene expression in human dental pulp stem cells and osteoblasts: Evidence for HDAC2 involvement[J]. Stem Cells, 2014, 32(1): 279-289. doi: 10.1002/stem.1544 [21] Li S, Li M, Liu X, et al. Genetic and chemical screenings identify HDAC3 as a key regulator in hepatic differentiation of human pluripotent stem cells[J]. Stem Cell Reports, 2018, 11(1): 22-31. doi: 10.1016/j.stemcr.2018.05.001 [22] Bao Y, Chen H, Cai Z, et al. Advanced glycation end products inhibit neural stem cell differentiation via upregulation of HDAC3 expression[J]. Brain Res Bull, 2020, 159: 1-8. doi: 10.1016/j.brainresbull.2020.03.001 [23] Jonason J H, Xiao G, Zhang M, et al. Post-translational regulation of Runx2 in bone and cartilage[J]. J Dent Res, 2009, 88(8): 693-703. doi: 10.1177/0022034509341629 [24] Man K, Lawlor L, Jiang L H, et al. The selective histone deacetylase inhibitor MI192 enhances the osteogenic differentiation efficacy of human dental pulp stromal cells[J]. Int J Mol Sci, 2021, 22(10): 5224. doi: 10.3390/ijms22105224 [25] Ozawa Y, Towatari M, Tsuzuki S, et al. Histone deacetylase 3 associates with and represses the transcription factor GATA-2[J]. Blood, 2001, 98(7): 2116-2123. doi: 10.1182/blood.V98.7.2116 [26] Janardhan H P, Milstone Z J, Shin M, et al. Hdac3 regulates lymphovenous and lymphatic valve formation[J]. J Clin Invest, 2017, 127(11): 4193-4206. doi: 10.1172/JCI92852 [27] Xu Y, Takahashi Y, Wang Y, et al. Downregulation of GATA-2 and overexpression of adipogenic gene-PPARgamma in mesenchymal stem cells from patients with aplastic Anemia[J]. Exp Hematol, 2009, 37(12): 1393-1399. doi: 10.1016/j.exphem.2009.09.005 [28] Yamane T, Kunisada T, Yamazaki H, et al. Sequential requirements for SCL/tal-1, GATA-2, macrophage colony-stimulating factor, and osteoclast differentiation factor/osteoprotegerin ligand in osteoclast development[J]. Exp Hematol, 2000, 28(7): 833-840. doi: 10.1016/S0301-472X(00)00175-2 [29] 任明诗, 丁羽, 李子涵, 等. 成骨细胞与破骨细胞相互调节作用的研究进展[J]. 中国药理学通报, 2022, 38(6): 822-827. -





 下载:
下载: