miR-16-5p Promotes Inflammation and Apoptosis in Oxygen-Glucose Deprivation Microglia Model by Mediating GPR30 Expression
-
摘要:
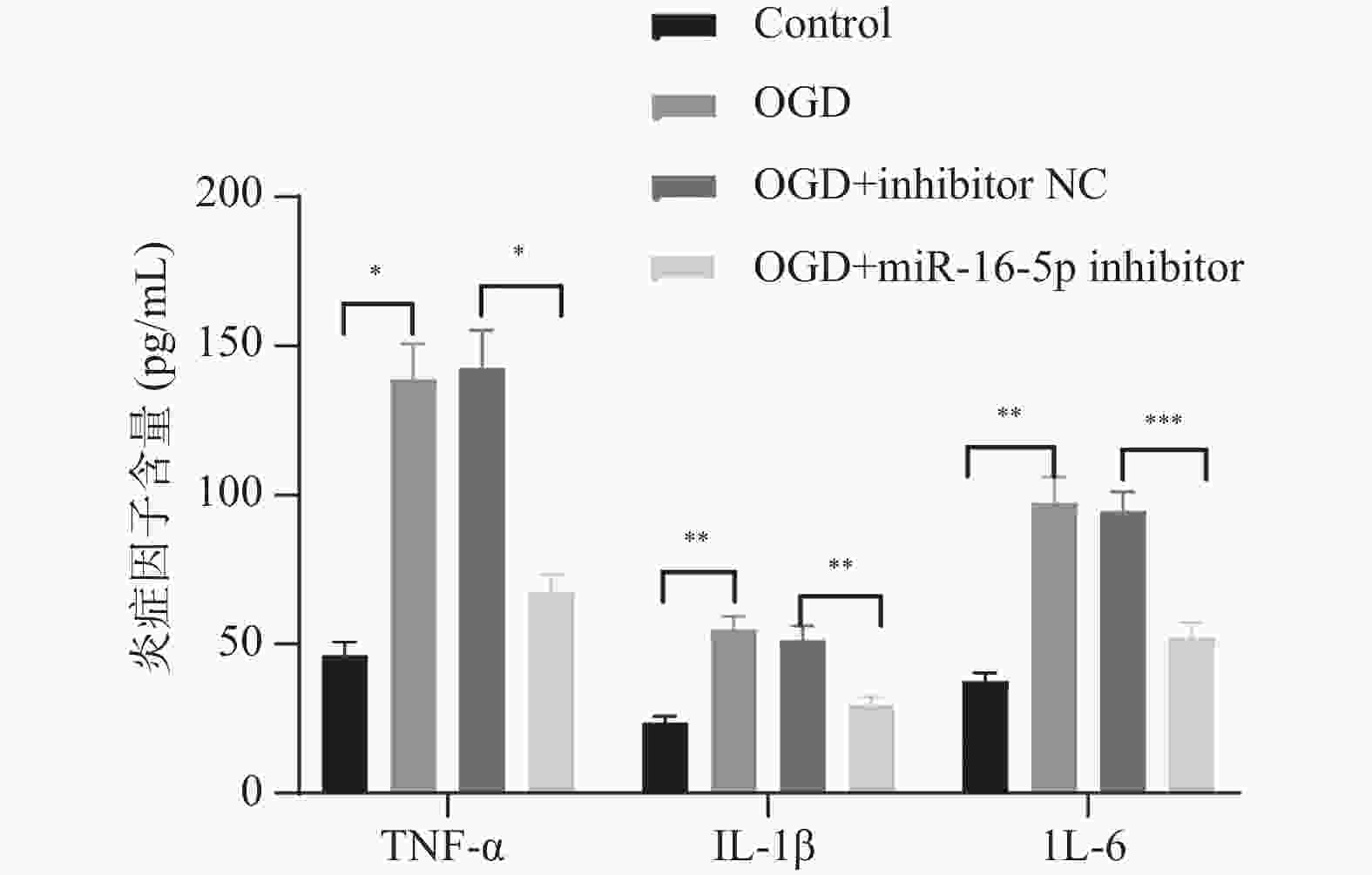
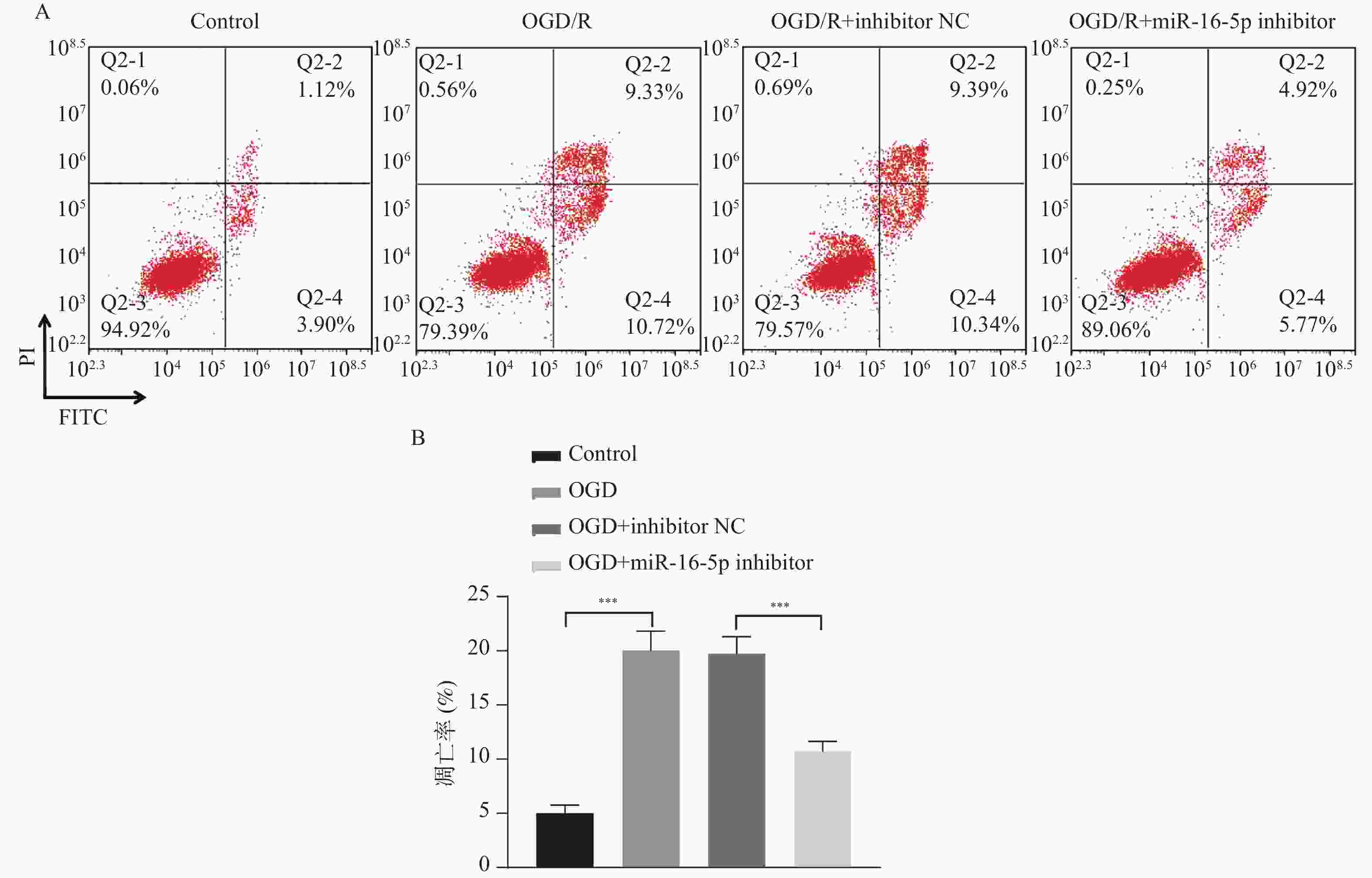
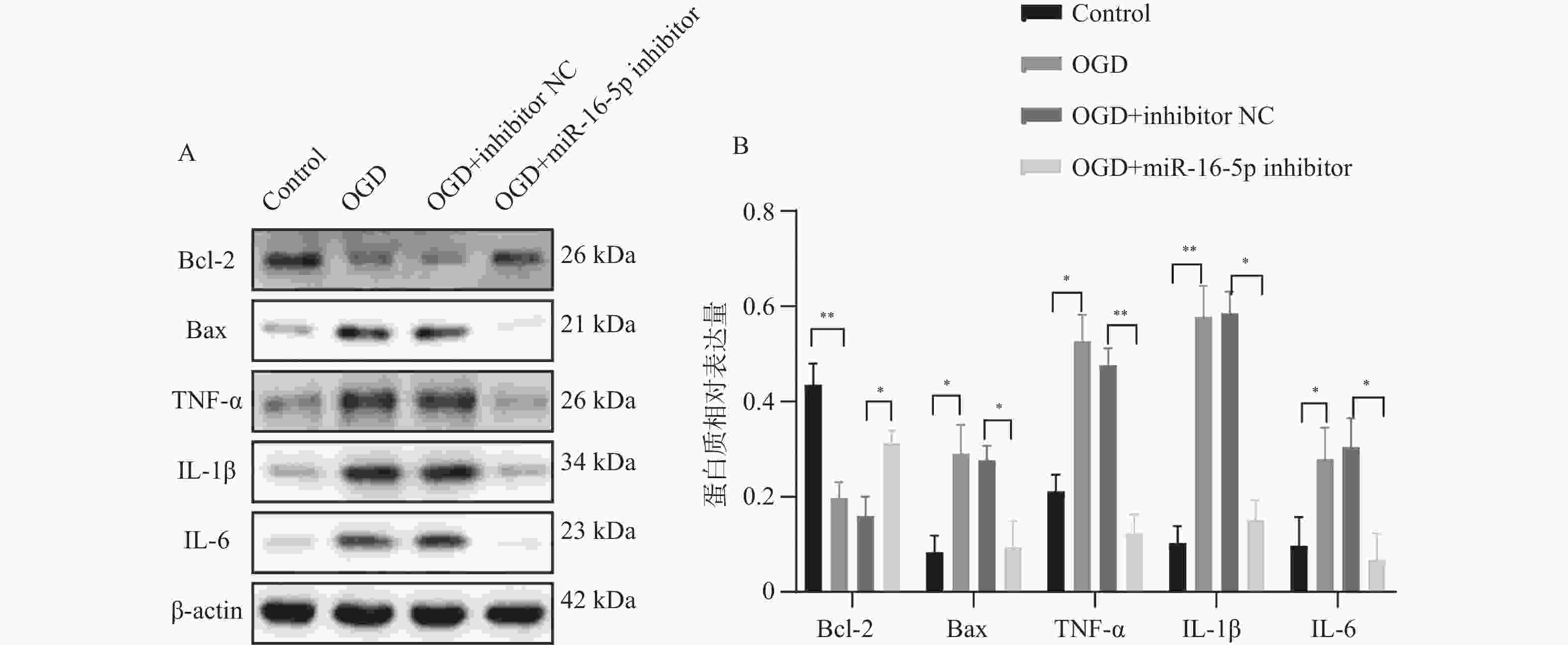
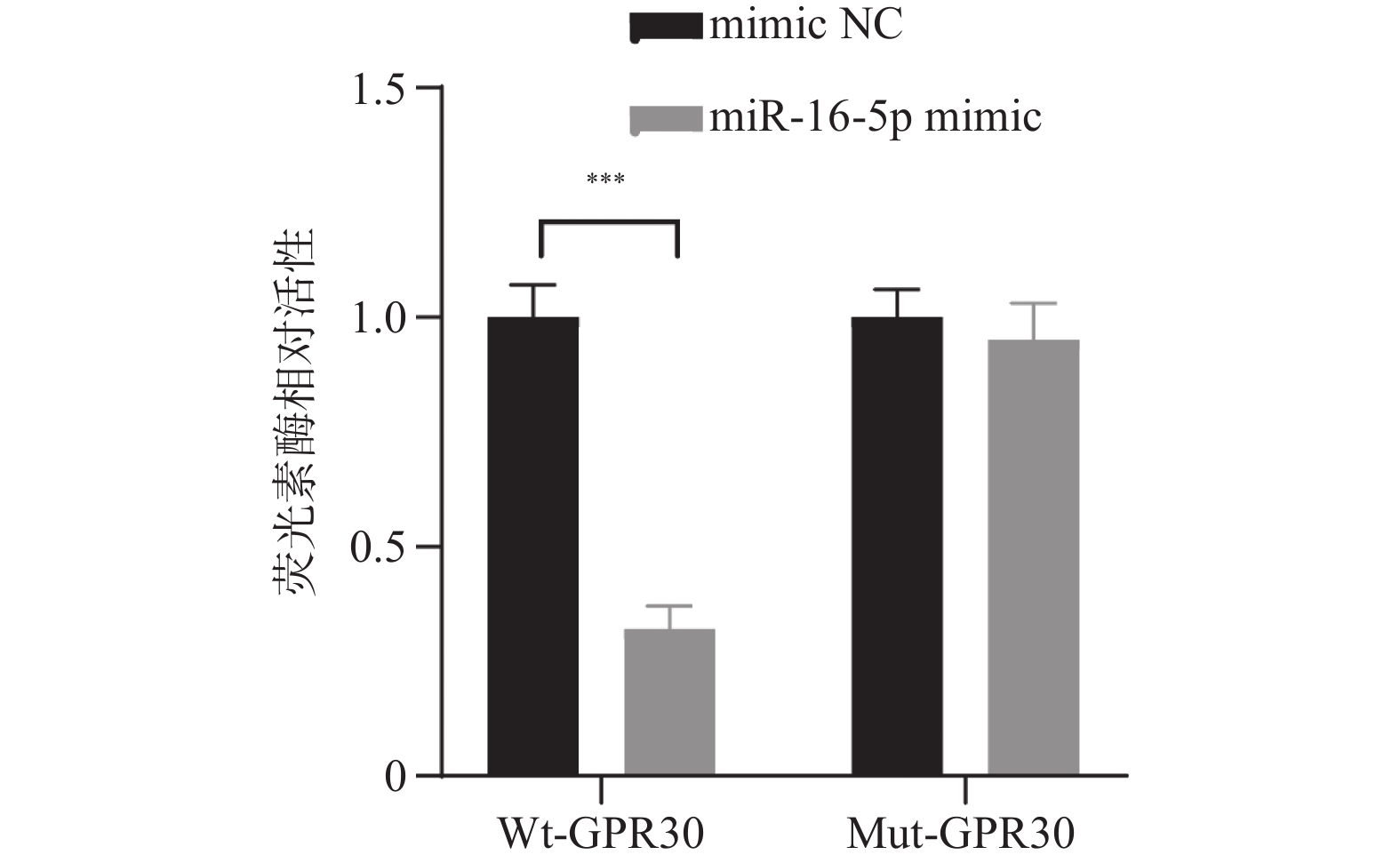
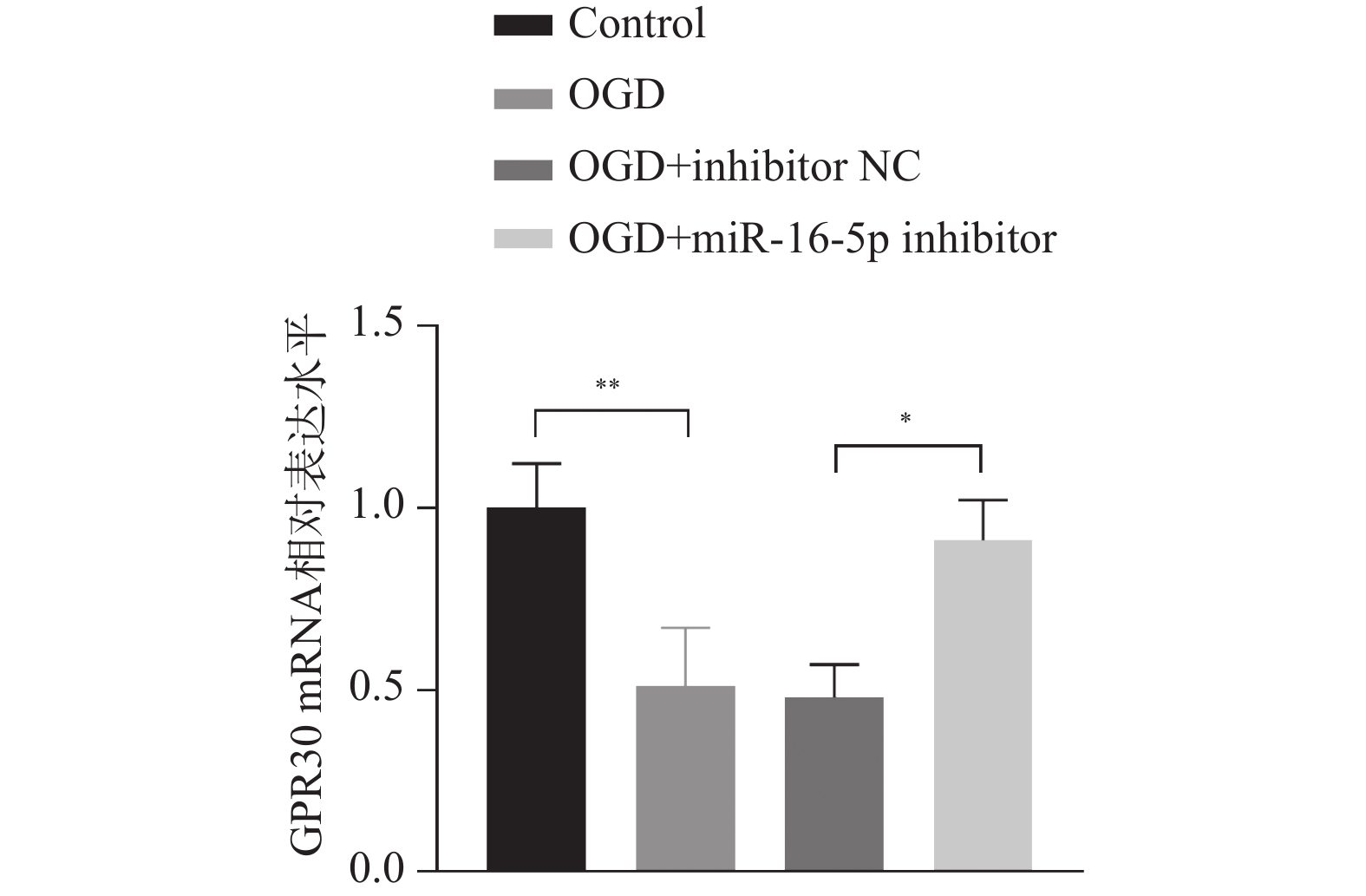
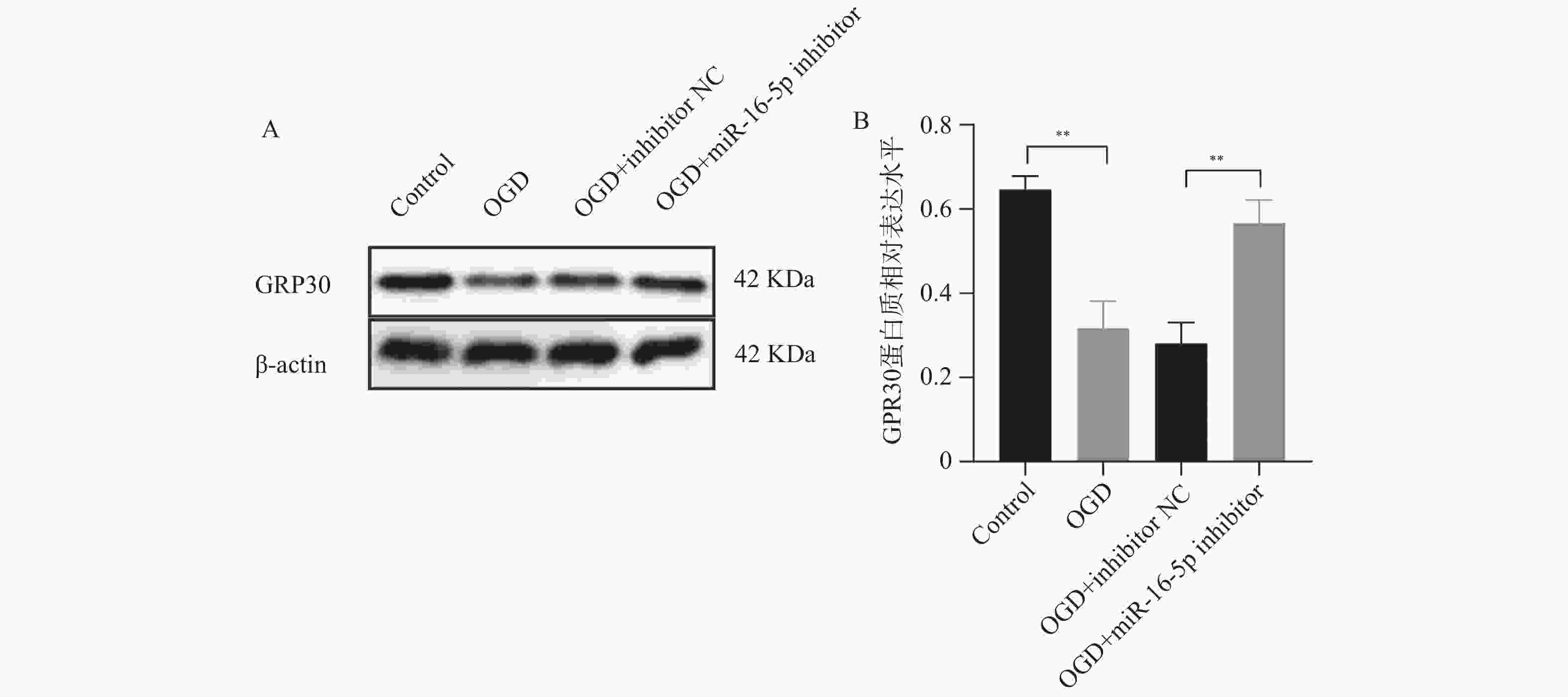
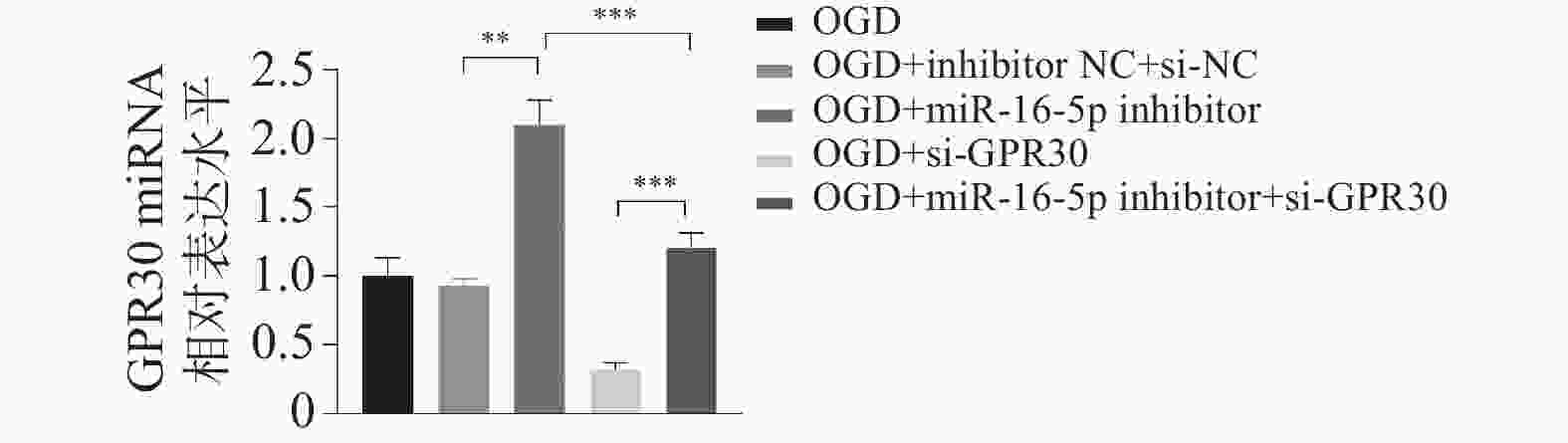
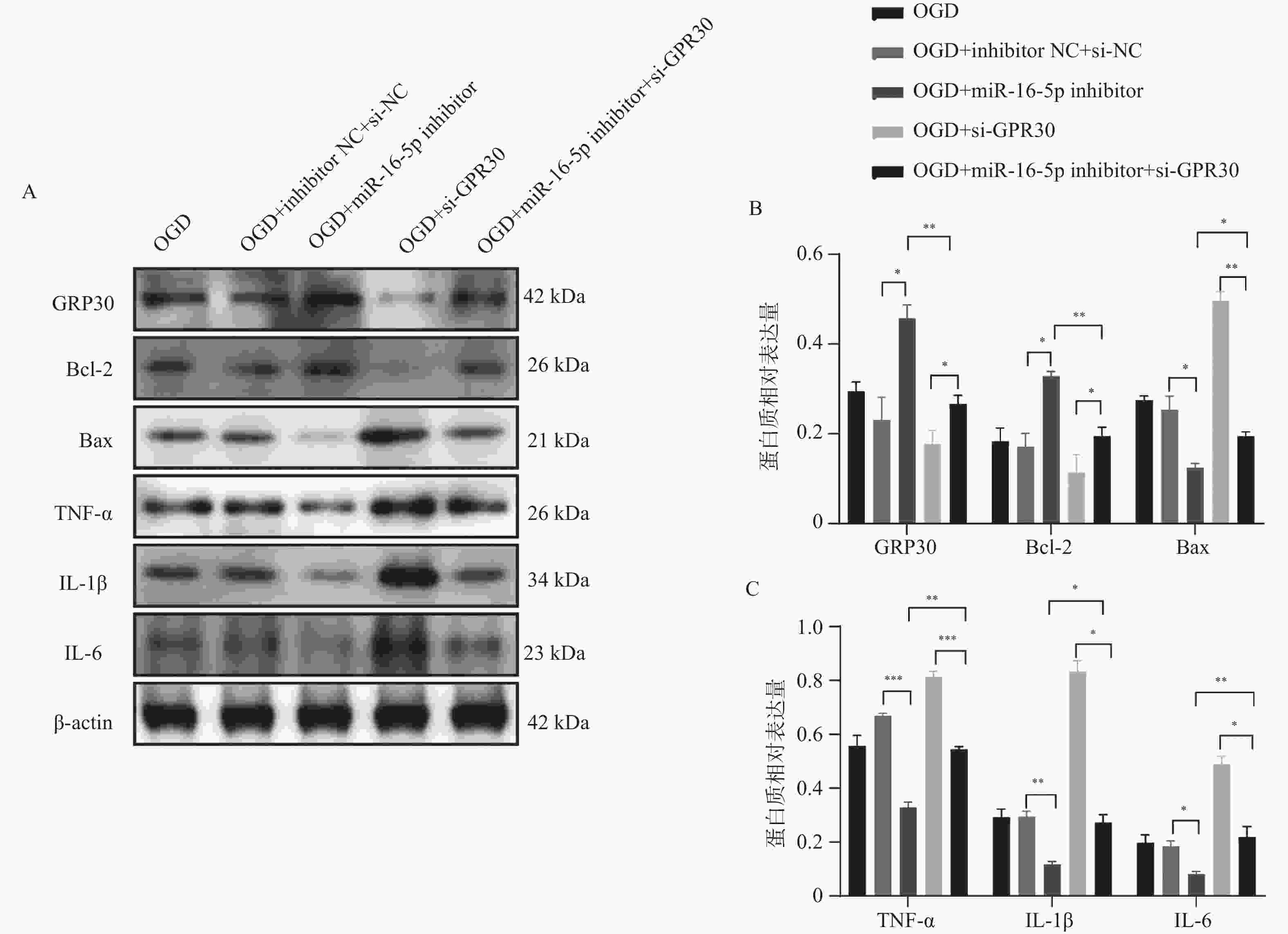
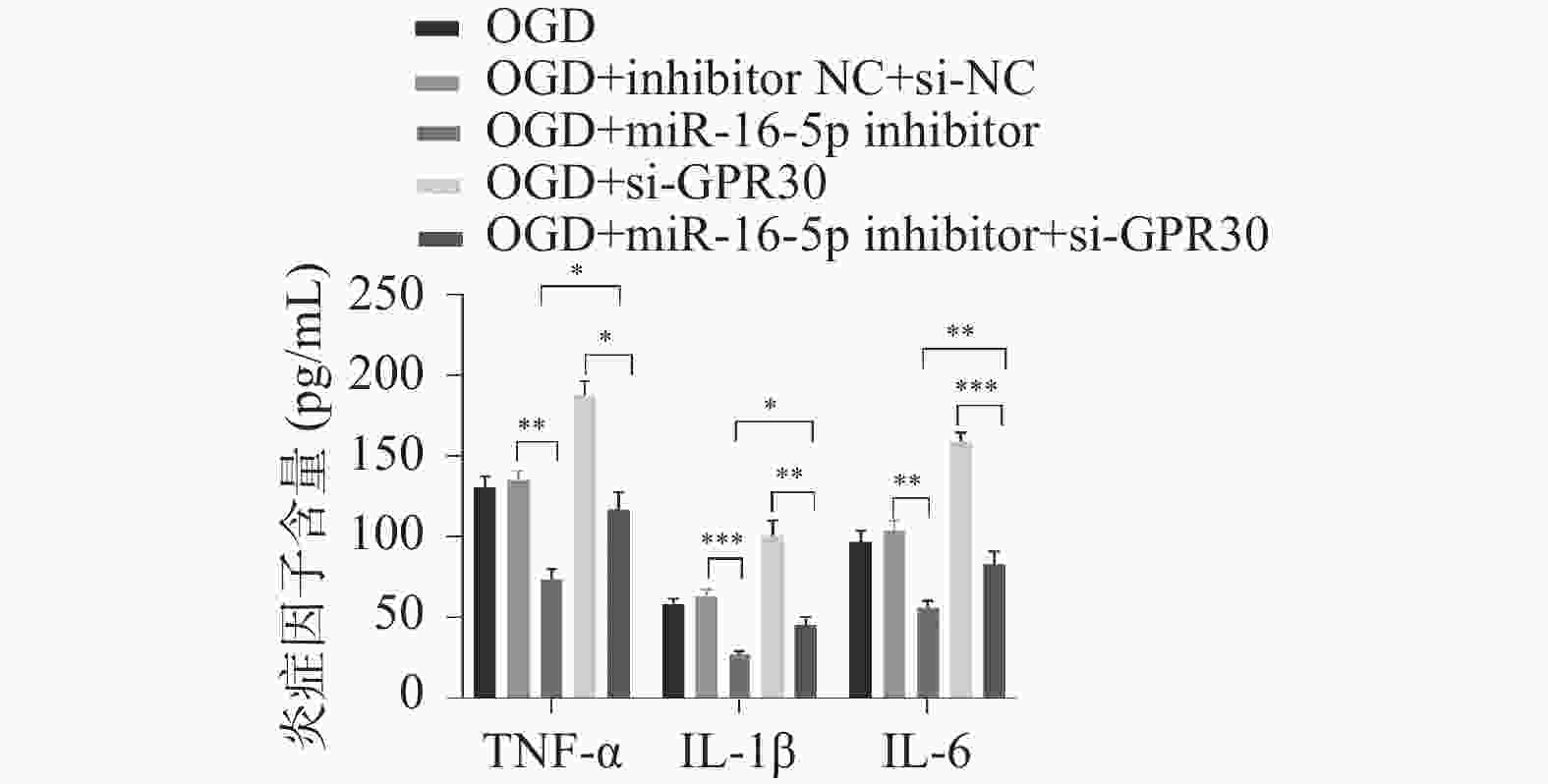
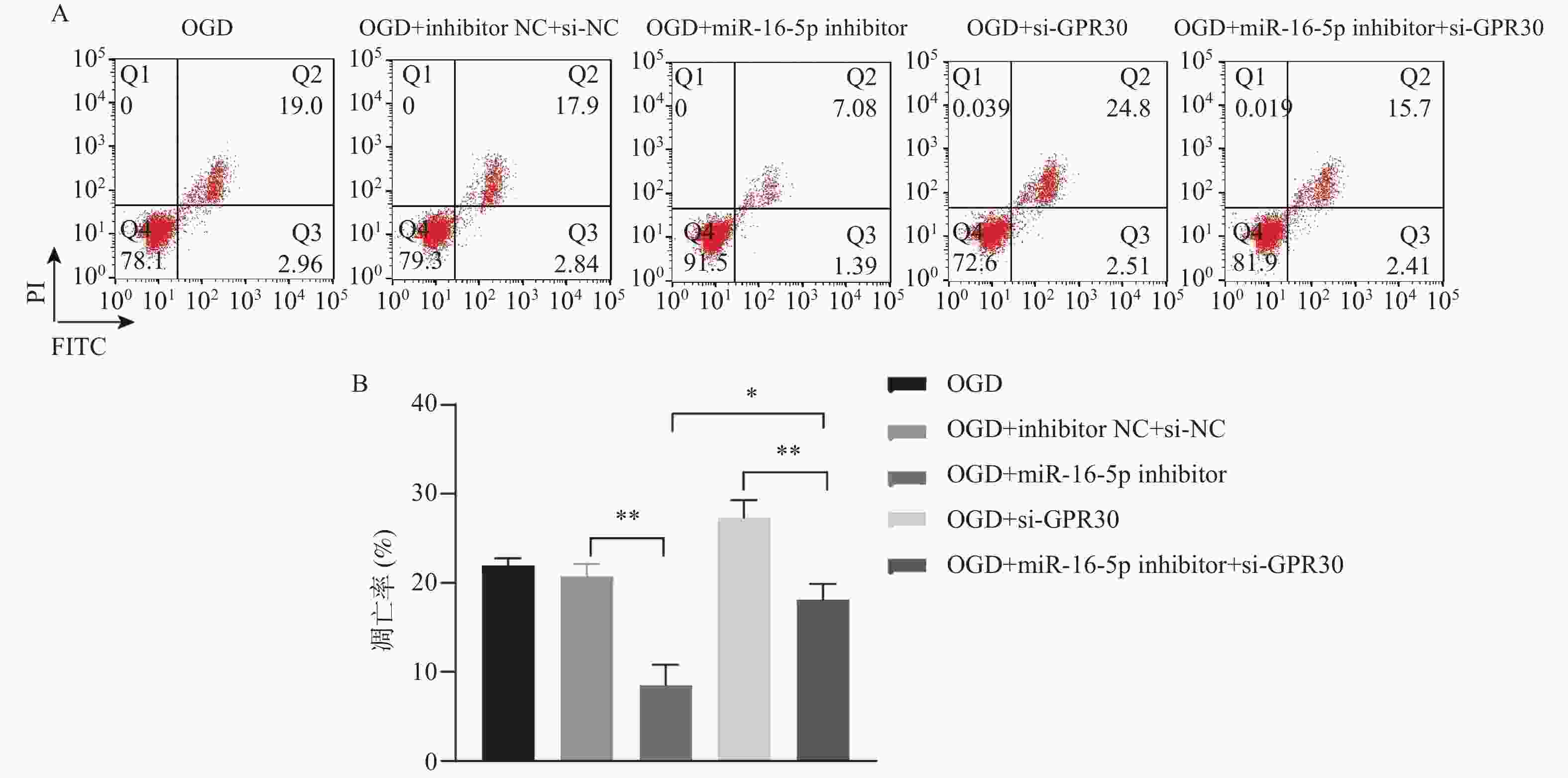
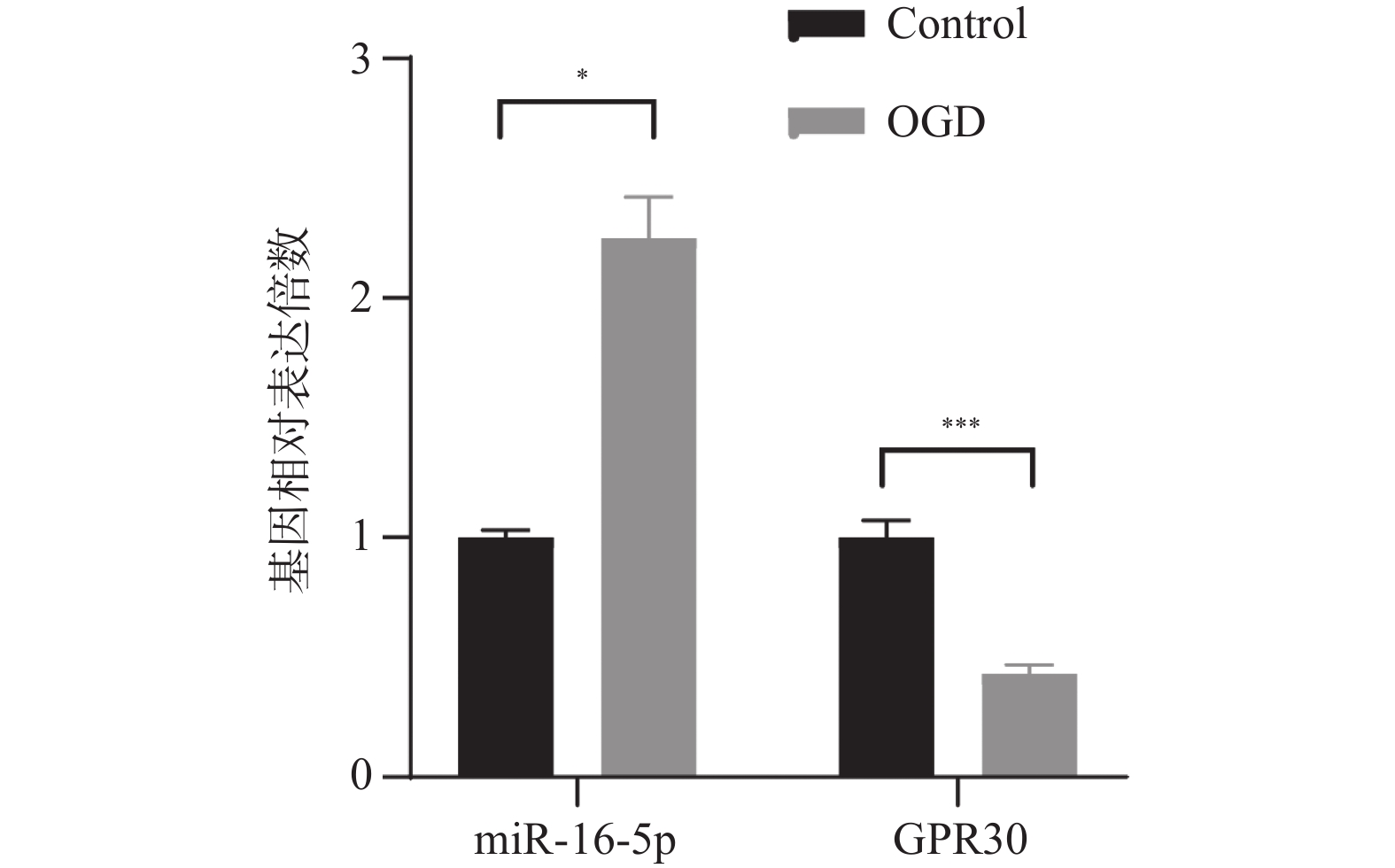
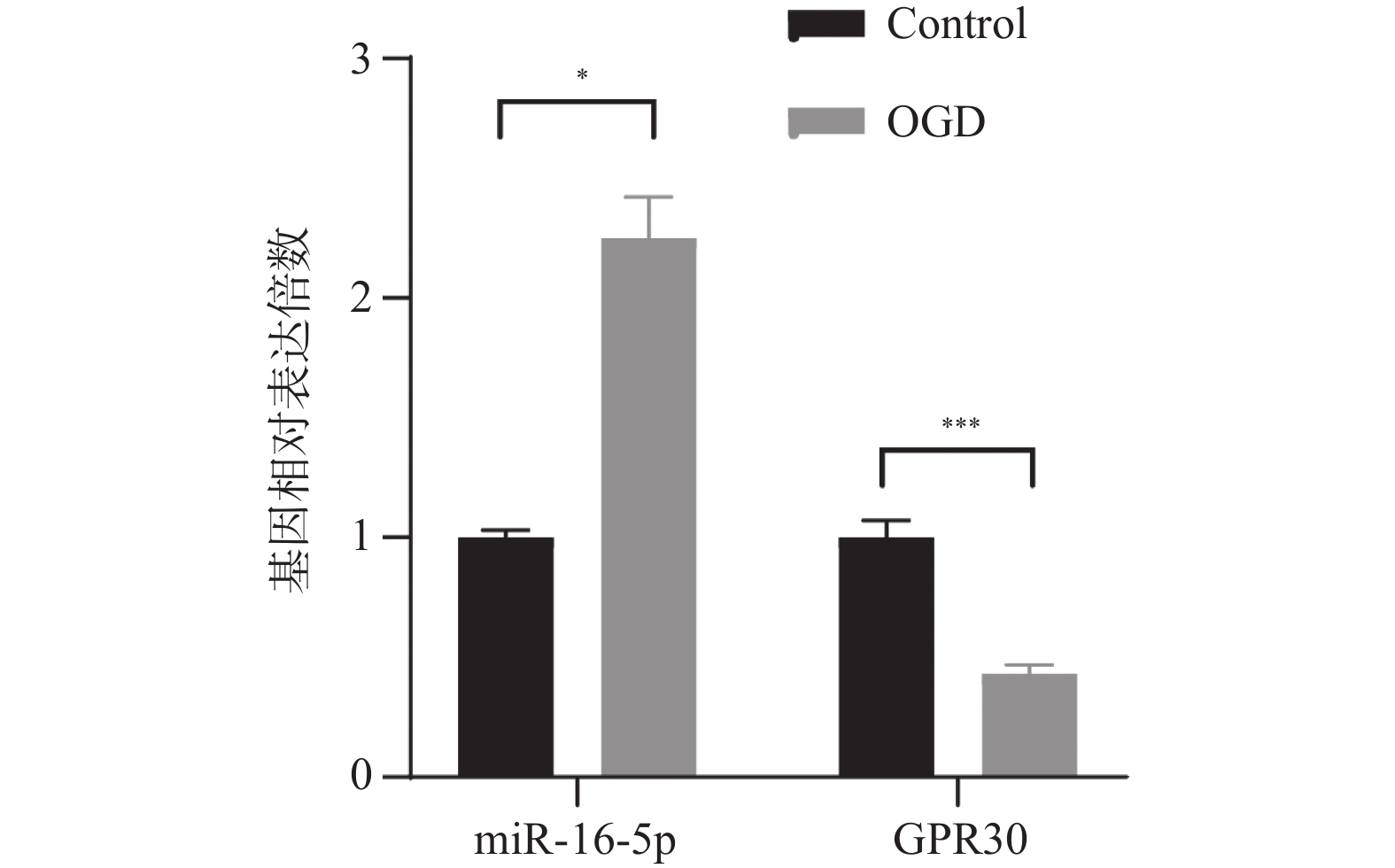
目的 探究在缺血性中风小胶质细胞(BV-2)体外模型中,miR-16-5p通过靶向GPR30表达促进细胞凋亡和炎症反应的分子机制。 方法 通过氧-葡萄糖剥夺(oxygen glucose deprivation,OGD)处理BV-2细胞构建缺血性中风的细胞模型。qRT-PCR评估OGD细胞中miR-16-5p和GPR30 mRNA水平。在OGD细胞中转染miR-16-5p inhibitor沉默miR-16-5p表达,并通过ELISA试剂盒和Annexin V-FITC/PI染色检测miR-16-5p对OGD细胞模型的炎症反应和凋亡的变化。通过Starbase预测和双荧光素酶报告基因实验验证miR-16-5p靶向GPR30的3’-非翻译区(UTR)序列。在OGD细胞中敲低miR-16-5p或/和GPR30检测细胞炎症反应和凋亡的变化。 结果 miR-16-5p在OGD诱导的细胞中表达升高(P < 0. 01),而GPR30表达降低(P < 0. 01)。敲低miR-16-5p抑制了OGD细胞的炎症因子表达水平以及细胞凋亡的比例(P < 0. 01)。抑制miR-16-5p表达能够上调GPR30 mRNA和蛋白质水平(P < 0. 01)。同时沉默miR-16-5p和GPR30,能够部分促进单独转染miR-16-5p inhibitor细胞的炎症因子表达水平和细胞凋亡的比例(P < 0. 05)。 结论 在小胶质细胞OGD模型中,miR-16-5p通过抑制GPR30的表达,激活小胶质细胞的炎症反应,促进其凋亡。 Abstract:Objective To explore the molecular mechanism of miR-16-5p promoting apoptosis and inflammatory response by targeting GPR30 expression in an in vitro ischemic stroke microglia model (BV-2). Methods An ischemic stroke cell model was established by subjecting BV-2 cells to oxygen-glucose deprivation (OGD). qRT-PCR was utilized to assess the levels of miR-16-5p and GPR30 mRNA in OGD cells. A miR-16-5p inhibitor was transfected into OGD cells to silence miR-16-5p expression, and alterations in inflammatory response and apoptosis were measured using ELISA kits and Annexin V-FITC/PI staining. Starbase was employed to predict interactions, and dual-luciferase reporter gene assays were conducted to confirm that miR-16-5p targets the 3'-untranslated region (UTR) sequence of GPR30. Changes in cellular inflammatory response and apoptosis were evaluated by knocking down miR-16-5p and/or GPR30 in OGD cells. Results miR-16-5p expression was significantly elevated (P < 0.01), while GPR30 expression was notably decreased (P < 0.01) in OGD-induced cells. Knockdown of miR-16-5p reduced the expression levels of inflammatory factors and the cell apoptosis ratio (P < 0.01). Inhibition of miR-16-5p expression led to an upregulation of GPR30 mRNA and protein levels (P < 0.01). Simultaneous silencing of both miR-16-5p and GPR30 partially enhanced inflammatory factor expression levels and the cell apoptosis ratio compared to cells transfected solely with the miR-16-5p inhibitor (P < 0.05). Conclusion In the microglia OGD model, miR-16-5p triggers inflammatory responses and enhances apoptosis by inhibiting GPR30 expression. -
Key words:
- Ischemic stroke /
- miR-16-5p /
- GPR30 /
- Inflammatory response /
- Apoptosis
-
表 1 qRT-PCR引物序列
Table 1. qRT-PCR primer sequences
正向引物序列(5'-3') 反向引物序列(5'-3') miR-16-5p GCAGCACGTAAATATTGGC GAACATGTCTGCGTATCTC GPR30 GCCACATAGTCAACCTTGCAGC CGTCTTCTGCTCCACATAGAGC U6 GAACGCCTCATGATTTGCAGG AGAAGACTGAAACAGCACAGAGA GAPDH CATCACTGCCACCCAGAAGACTG ATGCCAGTGAGCTTCCCGTTCAG -
[1] Gong Z, Guo J, Liu B, et al. Mechanisms of immune response and cell death in ischemic stroke and their regulation by natural compounds[J]. Front Immunol, 2023, 14: 1287857. [2] Khoshnam S E, Winlow W, Farzaneh M, et al. Pathogenic mechanisms following ischemic stroke[J]. Neurol Sci, 2017, 38(7): 1167-1186. doi: 10.1007/s10072-017-2938-1 [3] Kumari S, Dhapola R, Sharma P, et al. The impact of cytokines in neuroinflammation-mediated stroke[J]. Cytokine Growth Factor Rev, 2024, 78: 105-119. doi: 10.1016/j.cytogfr.2024.06.002 [4] Yang S, Qin C, Chen M, et al. TREM2-IGF1 mediated glucometabolic enhancement underlies microglial neuroprotective properties during ischemic stroke[J]. Adv Sci (Weinh), 2024, 11(10): e2305614. doi: 10.1002/advs.202305614 [5] Guan X, Zhu S, Song J, et al. Microglial CMPK2 promotes neuroinflammation and brain injury after ischemic stroke[J]. Cell Rep Med, 2024, 5(5): 101522. doi: 10.1016/j.xcrm.2024.101522 [6] Diener C, Keller A, Meese E. The miRNA-target interactions: An underestimated intricacy[J]. Nucleic Acids Res, 2024, 52(4): 1544-1557. doi: 10.1093/nar/gkad1142 [7] Diener C, Keller A, Meese E. Emerging concepts of miRNA therapeutics: From cells to clinic[J]. Trends Genet, 2022, 38(6): 613-626. doi: 10.1016/j.tig.2022.02.006 [8] Fehlmann T, Lehallier B, Schaum N, et al. Common diseases alter the physiological age-related blood microRNA profile[J]. Nat Commun, 2020, 11(1): 5958. doi: 10.1038/s41467-020-19665-1 [9] Peng S, Shen L, Yu X, et al. miR-200a attenuated oxidative stress, inflammation, and apoptosis in dextran sulfate sodium-induced colitis through activation of Nrf2[J]. Front Immunol, 2023, 14: 1196065. doi: 10.3389/fimmu.2023.1196065 [10] Macvanin M, Obradovic M, Zafirovic S, et al. The role of miRNAs in metabolic diseases[J]. Curr Med Chem, 2023, 30(17): 1922-1944. doi: 10.2174/0929867329666220801161536 [11] Li H, Duan J, Zhang T, et al. miR-16-5p aggravates sepsis-associated acute kidney injury by inducing apoptosis[J]. Ren Fail, 2024, 46(1): 2322688. doi: 10.1080/0886022X.2024.2322688 [12] Zhao Q C, Xu Z W, Peng Q M, et al. Enhancement of miR-16-5p on spinal cord injury-induced neuron apoptosis and inflammatory response through inactivating ERK1/2 pathway[J]. J Neurosurg Sci, 2024, 68(1): 101-108. [13] Hadjimarkou M M, Vasudevan N. GPER1/GPR30 in the brain: Crosstalk with classical estrogen receptors and implications for behavior[J]. J Steroid Biochem Mol Biol, 2018, 176: 57-64. doi: 10.1016/j.jsbmb.2017.04.012 [14] Zhang Z, Qin P, Deng Y, et al. The novel estrogenic receptor GPR30 alleviates ischemic injury by inhibiting TLR4-mediated microglial inflammation[J]. J Neuroinflammation, 2018, 15(1): 206. doi: 10.1186/s12974-018-1246-x [15] Kanazawa M, Ninomiya I, Hatakeyama M, et al. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke[J]. Int J Mol Sci, 2017, 18(10): 2135. doi: 10.3390/ijms18102135 [16] Wei W, Zhang L, Xin W, et al. TREM2 regulates microglial lipid droplet formation and represses post-ischemic brain injury[J]. Biomed Pharmacother, 2024, 170: 115962. doi: 10.1016/j.biopha.2023.115962 [17] Kolosowska N, Gotkiewicz M, Dhungana H, et al. Intracerebral overexpression of miR-669c is protective in mouse ischemic stroke model by targeting MyD88 and inducing alternative microglial/macrophage activation[J]. J Neuroinflammation, 2020, 17(1): 194. doi: 10.1186/s12974-020-01870-w [18] . Li G, Morris-Blanco K C, Lopez M S, et al. Impact of microRNAs on ischemic stroke: From pre- to post-disease[J]. Prog Neurobiol, 2018, 163-164(2): 59-78. [19] Yang X, Tang X, Sun P, et al. MicroRNA-15a/16-1 antagomir ameliorates ischemic brain injury in experimental stroke[J]. Stroke, 2017, 48(7): 1941-1947. doi: 10.1161/STROKEAHA.117.017284 [20] Tsai P C, Liao Y C, Wang Y S, et al. Serum microRNA-21 and microRNA-221 as potential biomarkers for cerebrovascular disease[J]. J Vasc Res, 2013, 50(4): 346-54. doi: 10.1159/000351767 [21] Peng H, Yang H, Xiang X, et al. MuicroRNA-221 participates in cerebral ischemic stroke by modulating endothelial cell function by regulating the PTEN/PI3K/AKT pathway[J]. Exp Ther Med, 2020, 19(1): 443-450. [22] Li P, Teng F, Gao F, et al. Identification of circulating microRNAs as potential biomarkers for detecting acute ischemic stroke[J]. Cell Mol Neurobiol, 2015, 35(3): 433-447. doi: 10.1007/s10571-014-0139-5 [23] Badacz R, Kleczynski P, Legutko J, et al. Expression of miR-1-3p, miR-16-5p and miR-122-5p as possible risk factors of secondary cardiovascular events[J]. Biomedicines, 2021, 9(8): 1055. doi: 10.3390/biomedicines9081055 [24] Toro R, Perez-Serra A, Mangas A, et al. miR-16-5p suppression protects human cardiomyocytes against endoplasmic reticulum and oxidative stress-induced injury[J]. Int J Mol Sci, 2022, 23(3): 1036. doi: 10.3390/ijms23031036 [25] Briz V, Liu Y, Zhu G, et al. A novel form of synaptic plasticity in field CA3 of hippocampus requires GPER1 activation and BDNF release[J]. J Cell Biol, 2015, 210(7): 1225-37. doi: 10.1083/jcb.201504092 [26] Day N L, Floyd C L, D'Alessandro T L, et al. 17beta-estradiol confers protection after traumatic brain injury in the rat and involves activation of G protein-coupled estrogen receptor 1[J]. J Neurotrauma, 2013, 30(17): 1531-41. doi: 10.1089/neu.2013.2854 [27] Wang X S, Yue J, Hu L N, et al. Activation of G protein-coupled receptor 30 protects neurons by regulating autophagy in astrocytes[J]. Glia, 2020, 68(1): 27-43. doi: 10.1002/glia.23697 -





 下载:
下载: