Effect of Autophagic Flux Impairment-mediated Apoptosis on Pressure Overload-induced Heart Failure
-
摘要:
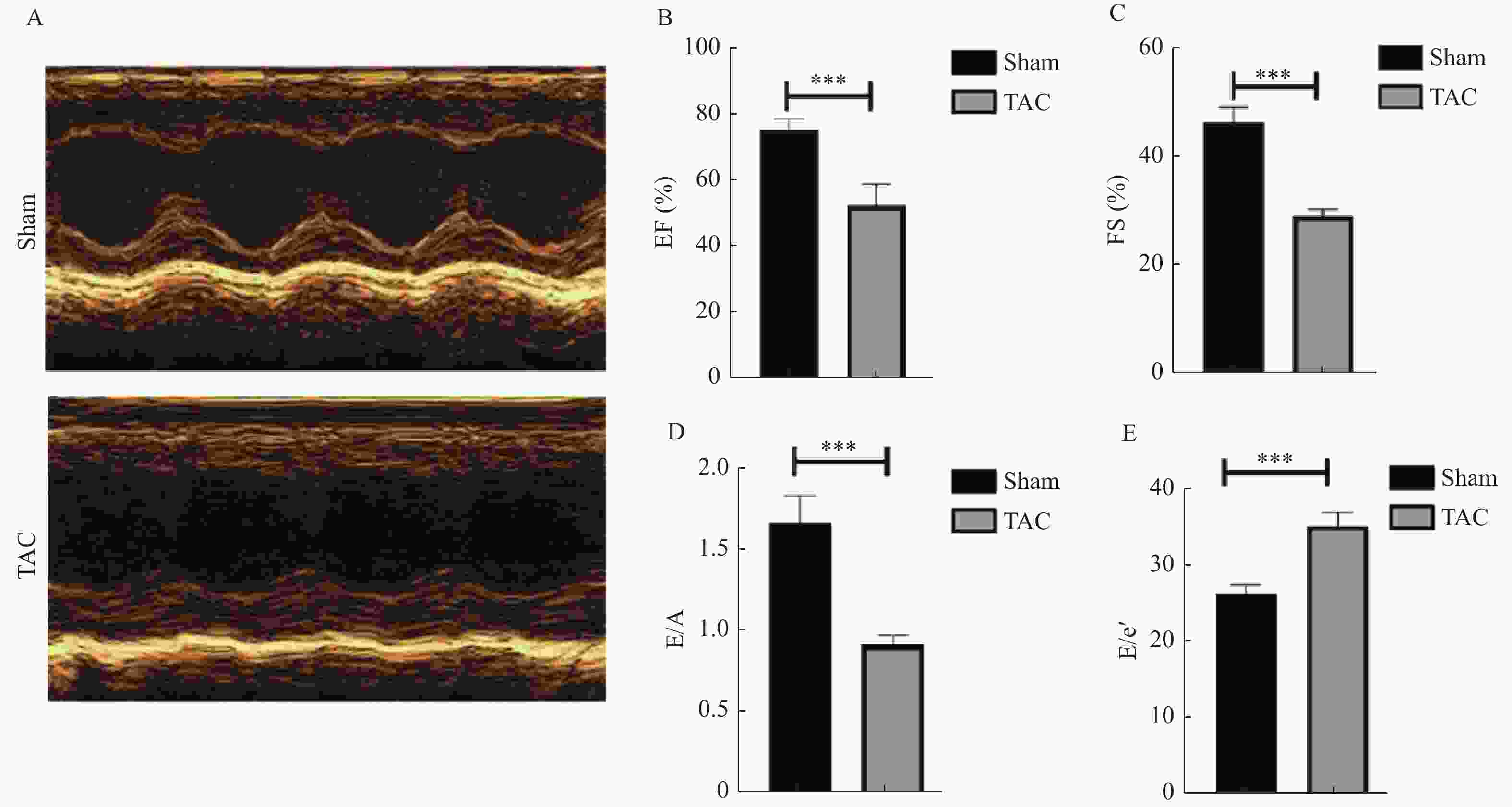
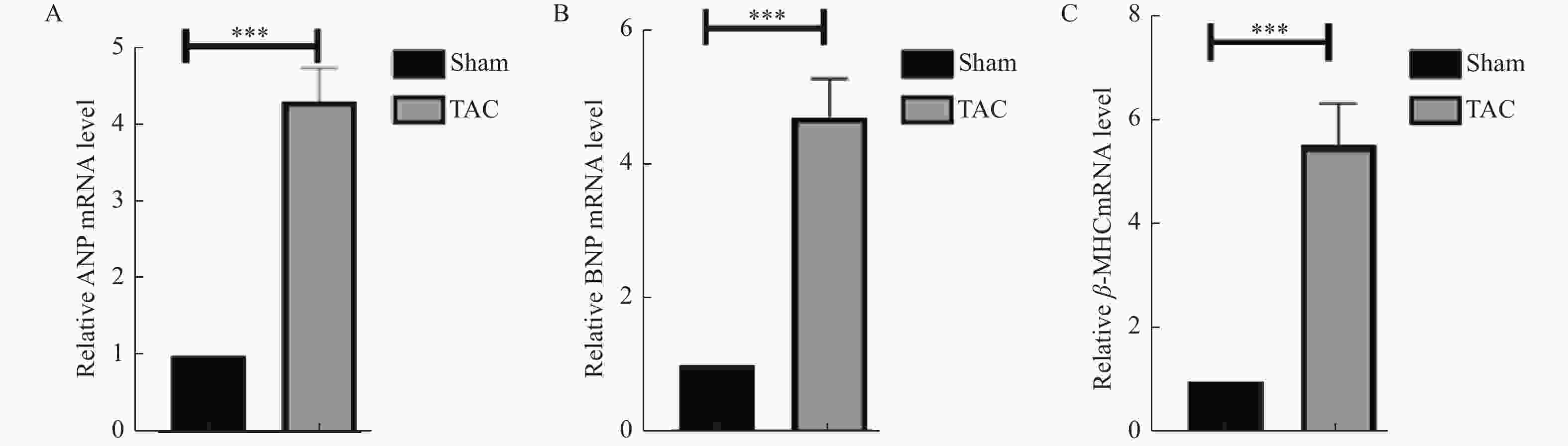
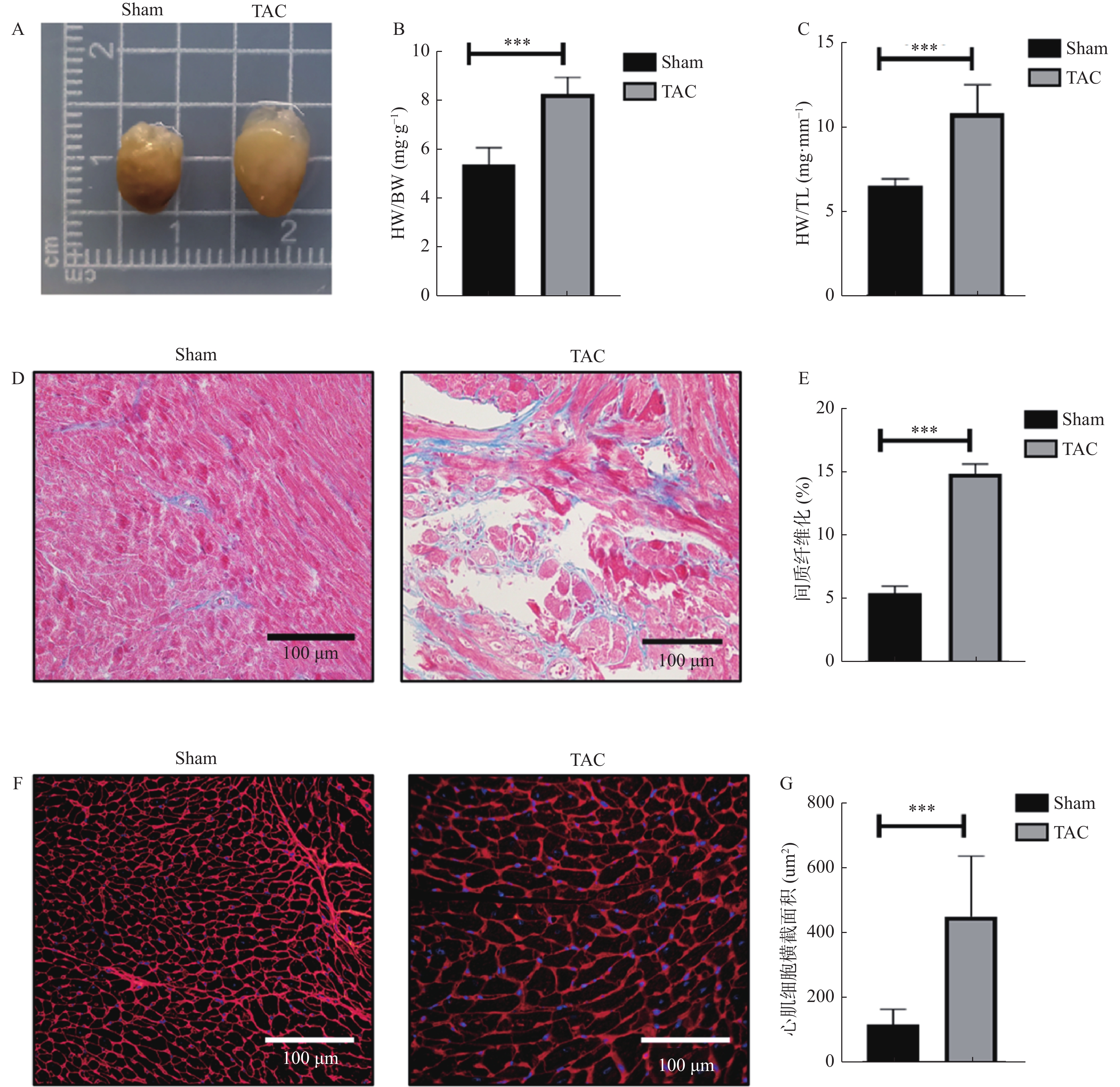
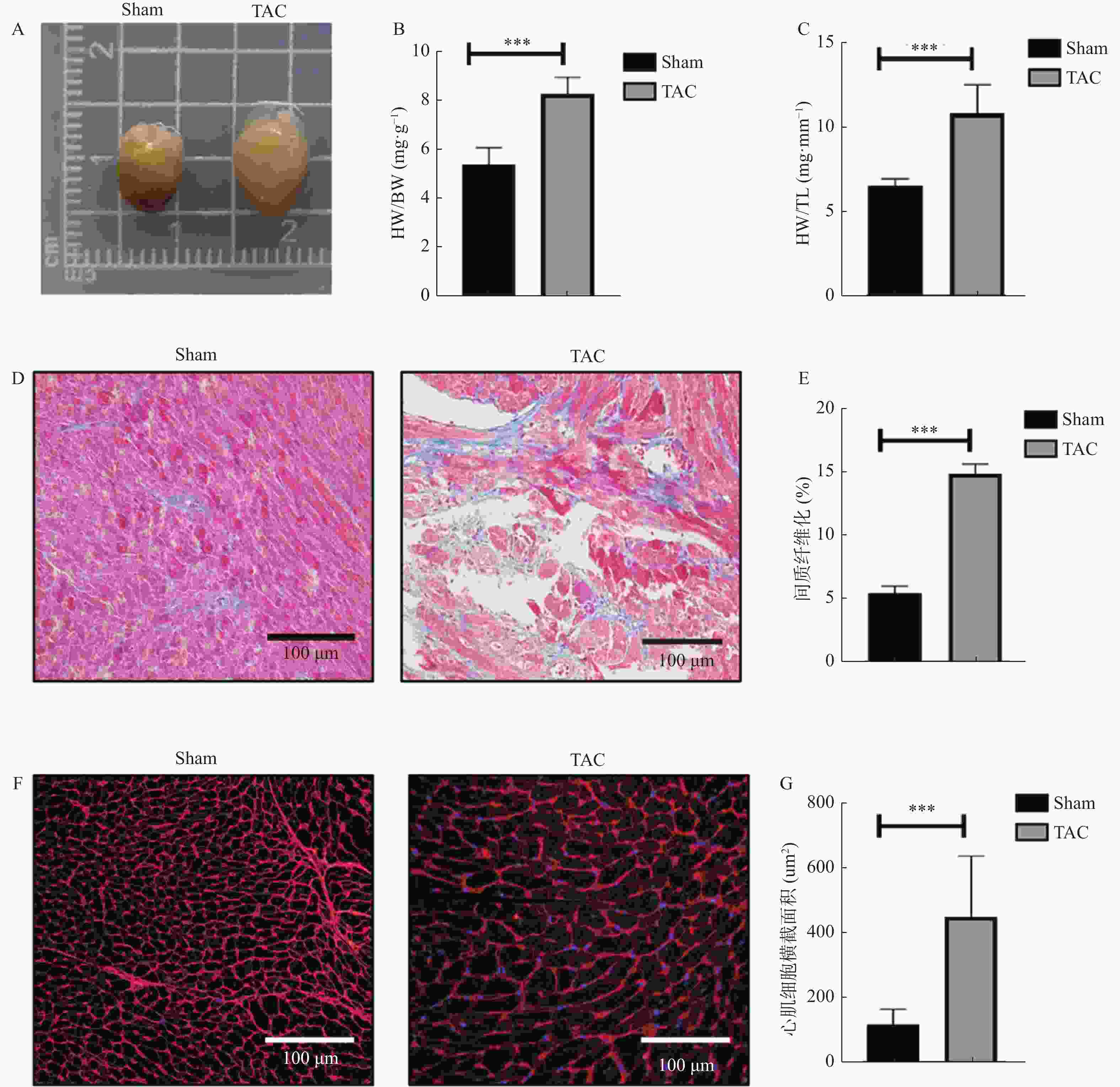
目的 探究自噬与细胞凋亡在压力超负荷诱导的心力衰竭(heart failure,HF)中的作用及机制。 方法 动物实验采用 C57/BL6J 小鼠20只(8~12周龄),通过横主动脉缩窄(transverse aortic constriction,TAC)建立压力超负荷诱导HF模型,随机分为对照组和模型组(n = 10)。术后4周行超声心动图检测心功能;计算心脏重量体重比(heart weight/body weight,HW/BW)及心脏重量胫骨长度比(heart weight/tibia length,HW/TL); Masson 和 WGA 染色评估组织病理学变化;实时荧光定量 PCR 检测心脏组织中 ANP、BNP 和 β-MHC 的 mRNA 表达; Western blot 检测自噬蛋白(Beclin1、P62、LC3-II/I)及细胞凋亡蛋白(BCL2、BAX、c-Caspase-3)的表达。以血管紧张素(angiotensin Ⅱ,AngⅡ)诱导 H9C2 心肌细胞构建体外HF模型,并用自噬抑制剂氯喹(chloroquine ,CQ)阻断自噬,随机分为对照组(PBS组)、模型组(AngⅡ 组)、PBS+CQ组、AngⅡ+CQ组。采用 Western blot 检测 Beclin1、P62、LC3-II/I、BAX、BCL2、c-Caspase-3 的表达情况。自噬双标慢病毒 mRFP-eGFP-LC3 检测细胞自噬流情况。 结果 与对照组相比,TAC 组小鼠心脏增大,HW/BW 和 HW/TL 值明显升高,射血分数(EF%)和缩短分数(FS%)降低(P < 0.001);纤维化及胶原沉积加重,心肌细胞横截面积增大(P < 0.001), ANP、BNP 和 β-MHC 的 mRNA (P < 0.001)表达水平升高,提示成功构建体内压力负荷诱导的 HF 模型。与对照组相比,TAC 组心肌组织自噬蛋白 Beclin1、P62、LC3-II/I(P < 0.01)表达上调,细胞凋亡蛋白 BAX、c-Caspase-3表达上调(P < 0.01),BCL-2(P < 0.001)蛋白表达降低。体外 HF 模型组中自噬小体明显增加,自噬溶酶体无明显变化,自噬通量受损。采用自噬流抑制 CQ 阻断自噬后,与 AngⅡ 组相比,加入 CQ 后自噬蛋白Beclin 1、LC3-II/I、P62升高(P < 0.05);自噬小体数量增多,自噬溶酶体无明显变化,自噬通量受损(P < 0.001);凋亡蛋白BAX、c-Caspase-3表达进一步上调(P < 0.01),而 BCL-2 蛋白表达水平降低(P < 0.001)。 结论 在压力超负荷诱导的HF模型中,自噬通量受阻可能通过增加心肌细胞凋亡参与压力超负荷诱导的心衰进展。 Abstract:Objective To explore the effect of autophagy and apoptosis on heart failure (HF) induced by pressure overload. Methods In the animal experiment, twenty C57/BL6J mice (aged 8-12 weeks) underwent transverse aortic constriction (TAC) to duplicate the model of pressure overload-induced HF. The mice were randomly divided into sham group (only threading was performed without ligation) and surgery group (TAC group). Four weeks after post-operation, echocardiography was used to assess cardiac function. Ratios of heart weight/body weight (HW/BW) and heart weight/tibia length (HW/TL) were calculated. Histopathological changes were assessed with Masson and WGA staining. Quantitative real-time PCR was used to quantify the mRNA levels of hypertrophy-related genes: ANP, BNP, and β-MHC. Western blot analysis was used to determine the expression of autophagy proteins (Beclin1, P62, LC3-II/I) and apoptosis proteins (BCL2, BAX, c-caspase-3). In the cell experiment, H9C2 cells were induced with angiotensin II (Ang II) to serve as an in vitro HF model. The H9C2 cells were divided into control (PBS group), Ang II group, PBS with chloroquine (PBS+CQ group), and Ang II with chloroquine (Ang II+CQ group). After modeling, western blotting was used to assay apoptosis protein expression (Beclin1, P62, LC3-II/I, BAX, BCL2, c-caspase-3). Autophagy double-labeled lentivirus mRFP-eGFP-LC3 was used to detect autophagic flux. Results Compared with the control group, the TAC group enlarged mouse heart, significantly increased HW/BW and HW/TL values, and decreased ejection fraction (EF%) and shortened fraction (FS%)(P < 0.001). Fibrosis and collagen deposition were aggravated, the cross-sectional area of cardiomyocytes increased(P < 0.001), and the mRNA expression levels of myocardial hypertrophy markers ANP, BNP and β-MHC (P < 0.001)were significantly increased, suggesting the successful construction of an understress-induced heart failure model in vivo. Compared to the control group, there was an upregulation of autophagy-related proteins Beclin1, P62, LC3-II/I(P < 0.01) and apoptosis proteins BAX, c-caspase-3(P < 0.01), while the expression of BCL-2(P < 0.001)protein was reduced. In the cell experiments, in the in vitro heart failure model group, the autophagosomes were significantly increased, but there was no significant change in autophagic lysosomes, and autophagic flux was impaired. After blocking the autophagy process with chloroquine (CQ), the Ang II+CQ group showed further increased expression of the autophagic proteins Beclin 1, LC3-II/I, P62(P < 0.05), and apoptosis proteins BAX and cleaved caspase-3(P < 0.01) compared to the Ang II group, and a further decrease in protein levels of BCL-2(P < 0.001). Additionally, CQ led to a significant increase in the number of autophagosomes, but there was no significant change in autophagic lysosomes, and autophagic flux was impaired. Conclusion Both autophagy and apoptosis are activated in pressure overload-induced heart failure, and impaired autophagic flux exacerbates apoptosis in this model. -
Key words:
- Heart failure /
- Transverse aortic constriction /
- Autophagy /
- Apoptosis /
- Cardiac remodeling
-
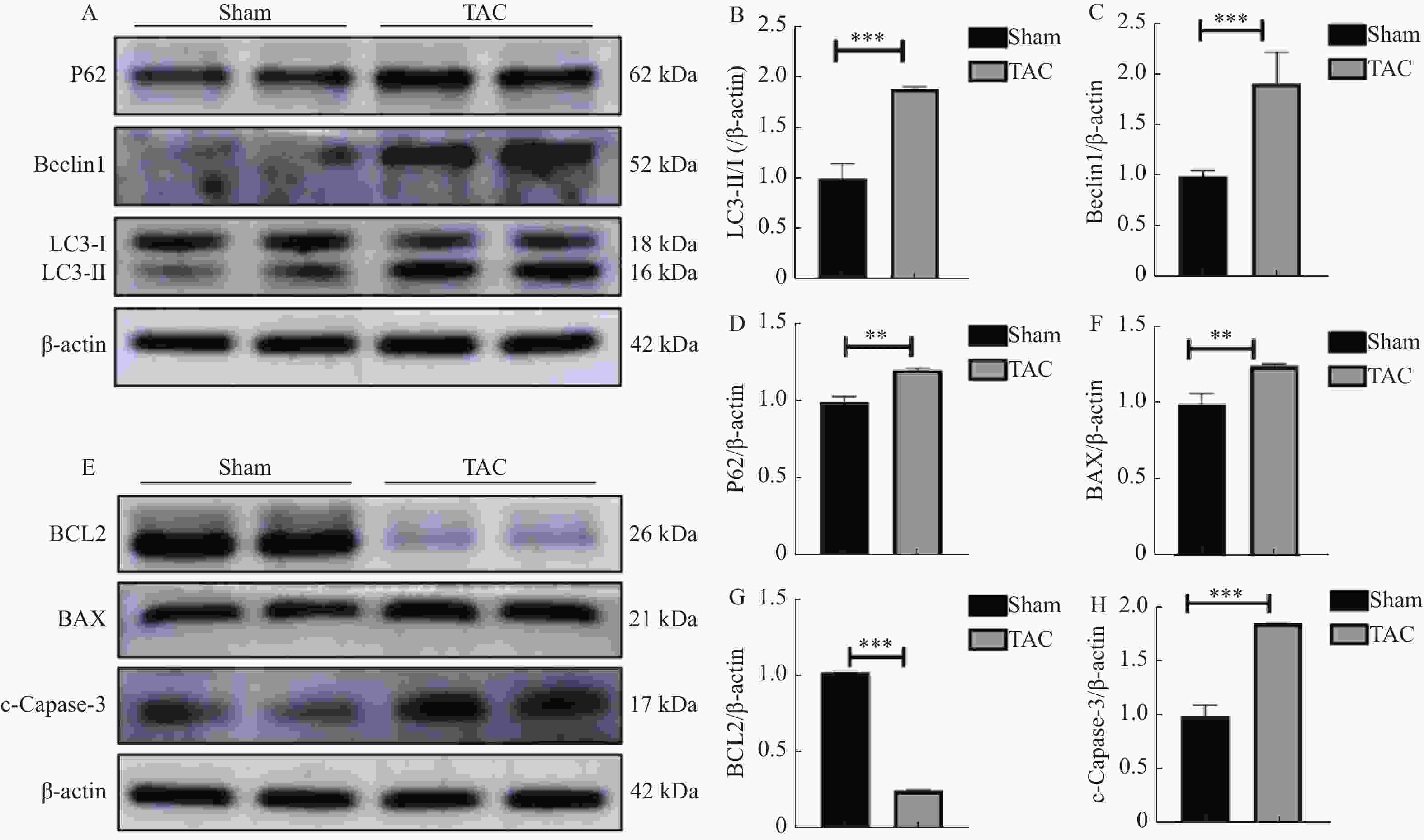
图 4 小鼠心肌自噬、凋亡相关蛋白表达比较[($\bar x \pm s $),n = 6 ]
A:自噬相关蛋白表达电泳图;B:LC3-II/I蛋白表达统计分析;C:Beclin1蛋白表达统计分析;D:P62蛋白表达统计分析;E:凋亡相关蛋白表达电泳图;F:BAX蛋白表达统计分析;G:BCL2蛋白表达统计分析;H:c-Capase-3蛋白表达统计分析;与对照组比较,**P < 0.01,***P < 0.001。
Figure 4. Comparison of myocardial autophagy and apoptosis-related protein expressions in mice [($\bar x \pm s $),n = 6 )]
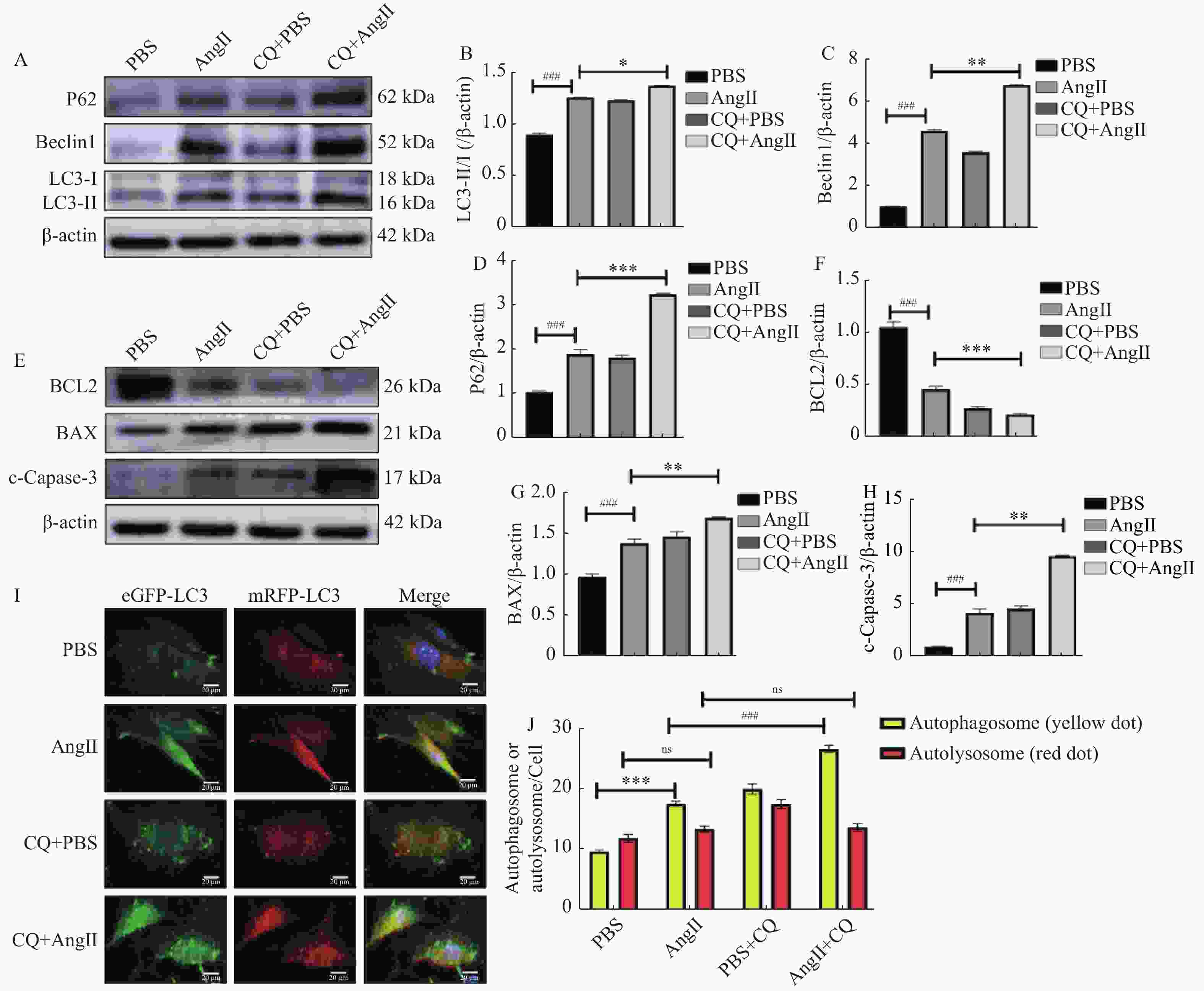
图 5 氯喹抑制自噬后凋亡相关蛋白的表达 [($\bar x \pm s $),n = 5 ]
A:自噬相关蛋白表达电泳图;B:P62蛋白表达统计分析;C:Beclin1蛋白表达统计分析;D:LC3-II/I蛋白表达统计分析;E:凋亡相关蛋白表达电泳图;F:BCL2 蛋白表达统计分析;G:BAX 蛋白表达统计分析;H:c-Capase-3 蛋白表达统计分析;I和J:自噬通量变化的代表性荧光图和统计分析,黄色小点代表自噬小体,红色小点代表自噬溶酶体(比例尺20 μm);与PBS组比较,##P < 0.01,###P < 0.001;与 AngⅡ 组比较,*P < 0.05,**P < 0.01,***P < 0.001。
Figure 5. Expression of apoptosis-related proteins after inhibition of autophagy by chloroquine [($\bar x \pm s $),n = 5)]
表 1 ANP、BNP 和 β-MHC 引物序列
Table 1. Primer sequences of ANP,BNP and β-MHC
基因 引物序列(5'→3') 引物
长度(bp)ANP F:TTCGGGGGTAGGATTGACAG
R:CACACCACAAGGGCTTAGGA20
20BNP F:TGTTTCTGCTTTTCCTTTATCTG
R:TCTTTTTGGGTGTTCTTTTGTGA23
23β-MHC F:CTCCTGCTGTTTCCTTACTTGC
R:CTCCCACATTCCTACGGCAC22
20β-actin F:CTGTCCCTGTATGCCTCTG
R:ATGTCACGCACGATTTCC19
18 -
[1] Savarese G, Becher P M, Lund L H, et al. Global burden of heart failure: A comprehensive and updated review of epidemiology[J]. Cardiovasc Res, 2023, 118(17): 3272-3287. doi: 10.1093/cvr/cvac013 [2] Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy[J]. Nat Rev Cardiol, 2018, 15(7): 387-407. doi: 10.1038/s41569-018-0007-y [3] Yang K, Shan X, Songru Y, et al. Network pharmacology integrated with experimental validation to elucidate the mechanisms of action of the Guizhi-Gancao decoction in the treatment of phenylephrine-induced cardiac hypertrophy[J]. Pharm Biol, 2024, 62(1): 456-471. doi: 10.1080/13880209.2024.2354335 [4] Ikeda S, Zablocki D, Sadoshima J. The role of autophagy in death of cardiomyocytes[J]. J Mol Cell Cardiol, 2022, 165: 1-8. [5] Rothermel B A, Hill J A. The heart of autophagy: Deconstructing cardiac proteotoxicity[J]. Autophagy, 2008, 4(7): 932-935. doi: 10.4161/auto.6756 [6] Nikoletopoulou V, Markaki M, Palikaras K, et al. Crosstalk between apoptosis, necrosis and autophagy[J]. Biochim Biophys Acta, 2013, 1833(12): 3448-3459. doi: 10.1016/j.bbamcr.2013.06.001 [7] Park W, Wei S, Kim B S, et al. Diversity and complexity of cell death: A historical review[J]. Exp Mol Med, 2023, 55(8): 1573-1594. doi: 10.1038/s12276-023-01078-x [8] Orogo A M, Gustafsson Å B. Cell death in the myocardium: My heart won't go on[J]. IUBMB Life, 2013, 65(8): 651-656. doi: 10.1002/iub.1180 [9] Mariño G, Niso-Santano M, Baehrecke E H, et al. Self-consumption: The interplay of autophagy and apoptosis[J]. Nat Rev Mol Cell Biol, 2014, 15(2): 81-94. doi: 10.1038/nrm3735 [10] Tang Y, Xu W, Liu Y, et al. Autophagy protects mitochondrial health in heart failure[J]. Heart Fail Rev, 2024, 29(1): 113-123. [11] Ecuypere J P, Parys J B, Bultynck G. Regulation of the autophagic bcl-2/beclin 1 interaction[J]. Cells, 2012, 1(3): 284-312. doi: 10.3390/cells1030284 [12] 张杨, 孙弯弯, 陆丽丹, 等. 细胞自噬与凋亡相互作用分子机制的研究进展[J]. 基础医学与临床, 2021, 41(9): 1342-1346. doi: 10.3969/j.issn.1001-6325.2021.09.019 [13] Gao G, Chen W, Yan M, et al. Rapamycin regulates the balance between cardiomyocyte apoptosis and autophagy in chronic heart failure by inhibiting mTOR signaling[J]. Int J Mol Med, 2020, 45(1): 195-209. [14] Wang K. Autophagy and apoptosis in liver injury[J]. Cell Cycle, 2015, 14(11): 1631-1642. doi: 10.1080/15384101.2015.1038685 [15] Yan X, Shang X, Feng Z, et al. Triterpenoid saponins of ilex pubescens against TNF-α induced inflammation and apoptosis in human umbilical vein endothelial cells via autophagy pathway[J]. J Pharm Pharmacol, 2022, 74(12): 1749-1757. doi: 10.1093/jpp/rgac074 [16] Kwon J, Kim J, Kim KI. Crosstalk between endoplasmic reticulum stress response and autophagy in human diseases[J]. Anim Cells Syst (Seoul), 2023, 27(1): 29-37. doi: 10.1080/19768354.2023.2181217 [17] Fernández A, Ordóñez R, Reiter R J, et al. Melatonin and endoplasmic reticulum stress: Relation to autophagy and apoptosis[J]. J Pineal Res, 2015, 59(3): 292-307. doi: 10.1111/jpi.12264 [18] Robin M, Issa A R, Santos C C, et al. Drosophila p53 integrates the antagonism between autophagy and apoptosis in response to stress[J]. Autophagy, 2019, 15(5): 771-784. doi: 10.1080/15548627.2018.1558001 [19] Li M, Su Y, Gao X, et al. Transition of autophagy and apoptosis in fibroblasts depends on dominant expression of HIF-1α or p53[J]. J Zhejiang Univ Sci B, 2022, 23(3): 204-217. doi: 10.1631/jzus.B2100187 [20] Pellegrini F R, De Martino S, Fianco G, et al. Blockage of autophagosome-lysosome fusion through SNAP29 O-GlcNAcylation promotes apoptosis via ROS production[J]. Autophagy, 2023, 19(7): 2078-2093. doi: 10.1080/15548627.2023.2170962 [21] Ai Y, Meng Y, Yan B, et al. The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death[J]. Mol Cell, 2024, 84(1): 170-179. doi: 10.1016/j.molcel.2023.11.040 [22] Li L, Xi R, Gao B, et al. Research progress of autophagy in heart failure[J]. Am J Transl Res, 2024, 16(5): 1991-2000. doi: 10.62347/OBXQ9477 [23] Corsetti G, Chen-Scarabelli C, Romano C, et al. Autophagy and oncosis/necroptosis are enhanced in cardiomyocytes from heart failure patients[J]. Med Sci Monit Basic Res, 2019, 25: 33-44. doi: 10.12659/MSMBR.913436 [24] Toda N, Sato T, Muraoka M, et al. Doxorubicin induces cardiomyocyte death owing to the accumulation of dysfunctional mitochondria by inhibiting the autophagy fusion process[J]. Free Radic Biol Med, 2023, 195: 47-57. doi: 10.1016/j.freeradbiomed.2022.12.082 [25] Li Z, Wang J, Yang X. Functions of autophagy in pathological cardiac hypertrophy[J]. Int J Biol Sci, 2015, 11(6): 672-678. doi: 10.7150/ijbs.11883 -





 下载:
下载: