-
摘要: 食品安全是全球重大的公共卫生问题,食源性病原菌是威胁人类健康及食品安全的主要因素,快速准确地检测食源性病原菌对保障食品安全、预防食源性疾病具有重要意义。传统病原微生物检测方法操作繁琐且耗时,不能及时检测出食品中的病原菌。随着生物技术的快速发展,其在食源性病原菌检测中的应用越来越广泛,为预防食源性疾病的发生及传播提供了强有力的技术支撑。综述常见食源性病原菌的危害以及快速检测方法的原理、应用及优缺点,以期为开展食品安全风险评估、食源性疾病监测等工作提供参考依据。Abstract: Food safety is a major public health problem in the world. Foodborne pathogens are the main factors threatening human health and food safety. Rapid and accurate detection of foodborne pathogens is of great significance to ensure food safety and prevent foodborne diseases. Traditional detection methods of pathogenic microorganisms are cumbersome and time-consuming, and can not detect pathogens in food in time. With the rapid development of biotechnology, its application in the detection of foodborne pathogens is more and more extensive, which provides a strong technical support for the prevention of the occurrence and spread of foodborne diseases. In this paper, the harm of common foodborne pathogens and the principle, application, advantages and disadvantages of rapid detection methods were reviewed, in order to provide reference for food safety risk assessment and foodborne disease monitoring.
-
Key words:
- Foodborne pathogens /
- Hazards /
- Detection technologies /
- Food safety
-
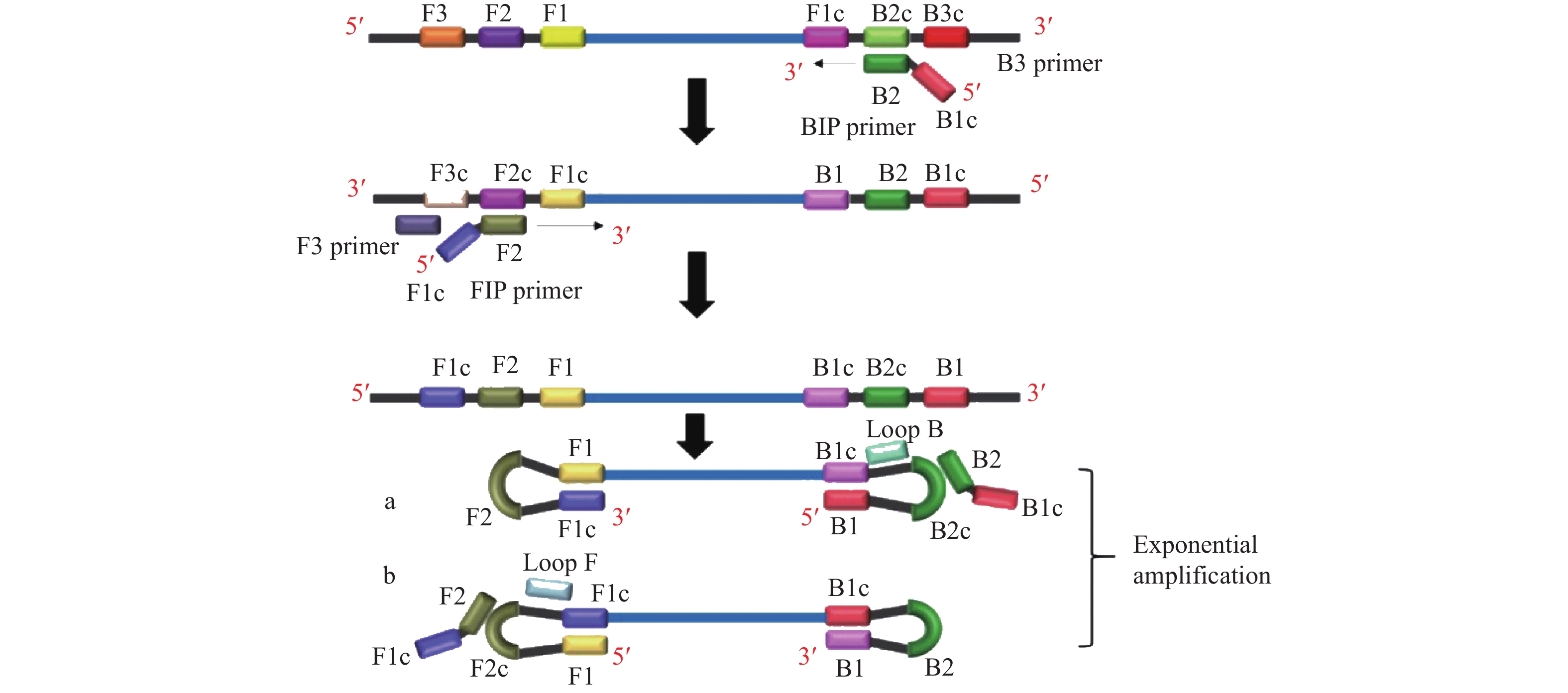
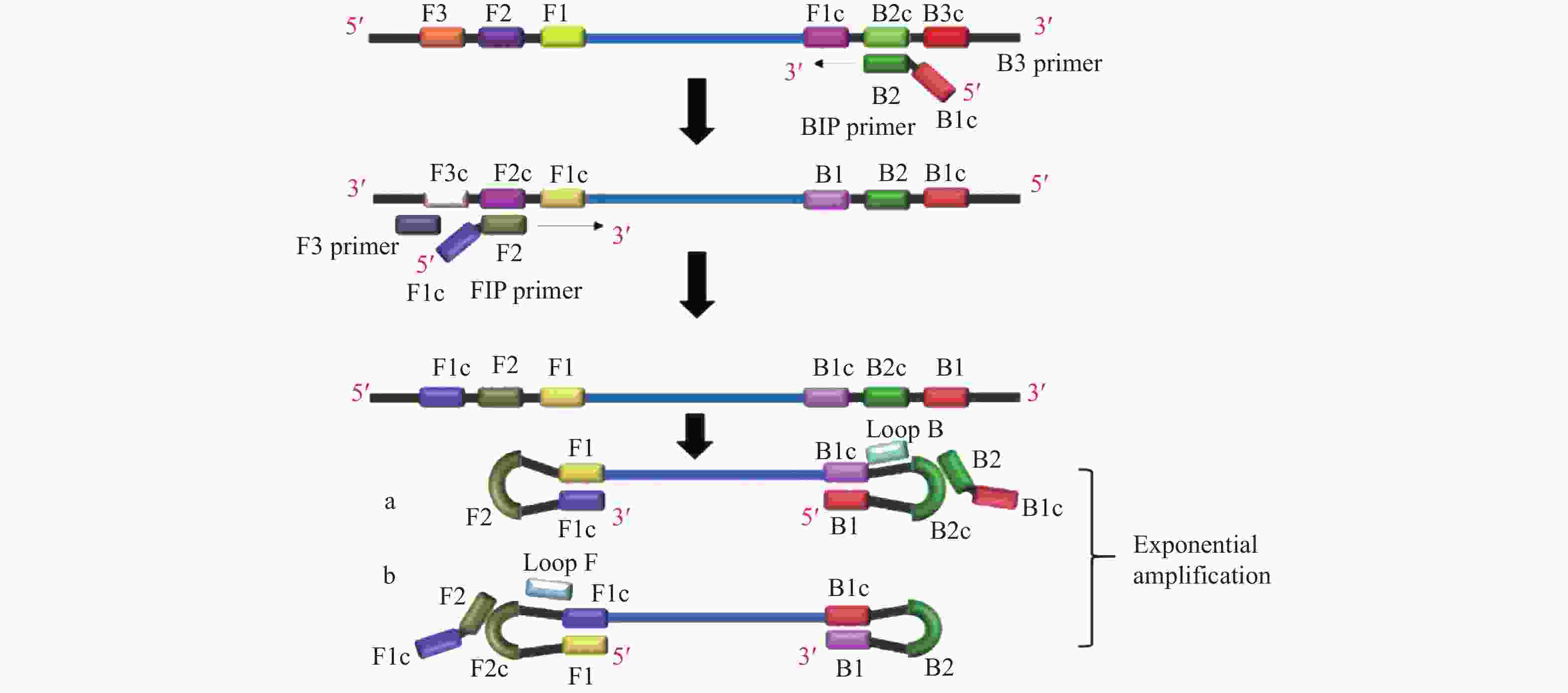
图 1 LAMP反应原理示意图[15]
Figure 1. Schematic diagram of loop-mediated isothermal amplification
表 1 常见食源性病原菌的主要食品传播介质及临床症状
Table 1. The main food transmission medium and clinical symptoms of common foodborne pathogens
表 2 LAMP、RCA及RPA 3种检测技术的比较
Table 2. A Comparison of three detection technologies: LAMP,RCA,and RPA
LAMP RCA RPA 聚合酶 Bst DNA聚合酶 phi29 DNA聚合酶 Bsu DNA聚合酶 扩增温度(℃) 60~65 37~65 37~40 引物数量(条) 4~6 1~2引物和PLP 2 目标基因长度(bp) <300 < 1700 <500 反应时间(min) <60 <150 20~40 表 3 食源性病原菌检测方法比较
Table 3. Comparison of detection methods for foodborne pathogens
检测方法 检测原理 优点 缺点 传统培养 根据病原菌的生长特性进行增菌、
培养、分离、纯化可培养分离得微生物 ①耗时、操作繁琐
②灵敏度低mPCR 加入2对以上引物,同时扩增多个目标基因 ①灵敏度和特异度好
②可同时检测多个微生物群①引物设计较为复杂
②可能产生引物二聚体qPCR 使用荧光染料或探针可定量监测反应中
PCR产物①高通量、自动化
②较普通PCR污染风险更低
③可实时进行定量分析①需要复杂的仪器设备
②不适合快速检测RT PCR 通过RNA创建互补DNA,对互补DNA进行定量 ①可进行定量分析
②可检测活的微生物①若mRNA降解则引起假阴性
②操作复杂,费用较高LAMP 根据目标基因的6个区域设计4种特异引物,
用Bst DNA聚合酶完成扩增①产物产量高
②不需复杂的热循环仪器
③可视化观察检测结果①LAMP产物不易降解
②可视化观察存在主观性
③凝胶电泳不能识别条带大小
④限制靶DNA长度<300 bpsRPA 重组酶、聚合酶参与 反应温度低、反应快速 引物设计难度高 CRISPR-Cas 基因编辑 高效的基因编辑技术 ①载体功能受病原菌基因大小限制
②引物设计范围小ELISA 抗原、抗体特异性结合 ①自动化,灵敏度和特异度好
②可一次处理大量样品仪器设备、操作复杂 代谢组学 基于代谢特征鉴定代谢产物 可对微生物进行定量 ①生物体代谢变化快,稳定性差
②数据分析专业性强传感器/
基因芯片物理、化学信号转换生物信息/核酸杂交 ①高通量、自动化
②灵敏度特异度高设备复杂 -
[1] Ribot E M,Hise K B. Future challenges for tracking foodborne diseases: Pulsenet,a 20-year-old US surveillance system for foodborne diseases,is expanding both globally and technologically[J]. Embo Rep,2016,17(11):1499-1505. doi: 10.15252/embr.201643128 [2] European Food Safety Authority, European Centre for Disease Prevention and Control. The European union one health 2021 zoonoses report[J]. Efsa J,2022,20(12):e07666. [3] Khalil I A,Troeger C,Blacker B F,et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The global burden of disease study 1990-2016[J]. Lancet Infect Dis,2018,18(11):1229-1240. doi: 10.1016/S1473-3099(18)30475-4 [4] Denamur E,Clermont O,Bonacorsi S,et al. The population genetics of pathogenic Escherichia coli[J]. Nat Rev Microbiol,2021,19(1):37-54. doi: 10.1038/s41579-020-0416-x [5] 田牧雨,张一敏,董鹏程,等. 沙门氏菌和单增李斯特菌诱导性耐酸响应机制的研究进展[J]. 食品科学,2019,40(5):316-322. [6] Qi X L,Wang H X,Bu S R,et al. Incidence rates and clinical symptoms of Salmonella,Vibrio parahaemolyticus,and Shigella infections in China,1998-2013[J]. J Infect Dev Ctries,2016,10(2):127-133. doi: 10.3855/jidc.6835 [7] Tong S Y,Davis J S,Eichenberger E,et al. Staphylococcus aureus infections: Epidemiology,pathophysiology,clinical manifestations,and management[J]. Clin Microbiol Rev,2015,28(3):603-661. doi: 10.1128/CMR.00134-14 [8] Boukharouba A,Gonzalez A,Garcia-ferrus M,et al. Simultaneous detection of four main foodborne pathogens in ready-to-eat food by using a simple and rapid multiplex PCR (mPCR) assay[J]. Int J Environ Res Public Health,2022,19(3):1031. doi: 10.3390/ijerph19031031 [9] Feng Y,Yau H,Chen S,et al. Rapid detection of Hypervirulent serovar 4h Listeria monocytogenes by multiplex PCR[J]. Front Microbiol,2020,11(6):1309. [10] Fusco V,Quero G M,Morea M,et al. Rapid and reliable identification of Staphylococcus aureus harbouring the enterotoxin gene cluster (egc) and quantitative detection in raw milk by real time PCR[J]. Int J Food Microbiol,2011,144(3):528-537. doi: 10.1016/j.ijfoodmicro.2010.11.016 [11] Hu Q,Lyu D,Shi X,et al. A modified molecular beacons-based multiplex real-time PCR assay for simultaneous detection of eight foodborne pathogens in a single reaction and its application[J]. Foodborne Pathog Dis,2014,11(3):207-214. doi: 10.1089/fpd.2013.1607 [12] Chen M,Lan X,Zhu L,et al. PCR mediated nucleic acid molecular recognition technology for detection of viable and dead foodborne pathogens[J]. Foods,2022,11(17):2675. doi: 10.3390/foods11172675 [13] Đermic D,Ljubic S,Matulic M,et al. Reverse transcription-quantitative PCR (RT-qPCR) without the need for prior removal of DNA[J]. Sci Rep,2023,13(1):11470. doi: 10.1038/s41598-023-38383-4 [14] Silva S,Pardee K,Pena L. Loop-mediated isothermal amplification (LAMP) for the diagnosis of Zika virus: A review[J]. Viruses,2019,12(1):19. doi: 10.3390/v12010019 [15] Wong Y P,Othman S,Lau Y L,et al. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms[J]. J Appl Microbiol,2018,124(3):626-643. doi: 10.1111/jam.13647 [16] Soroka M,Wasowicz B,Rymasezwska A. Loop-mediated isothermal amplification (LAMP): The better sibling of PCR[J]. Cells,2021,10(8):1931. doi: 10.3390/cells10081931 [17] Parida M,Sannarangaiah S,Dash P K,et al. Loop mediated isothermal amplification (LAMP): A new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases[J]. Rev Med Virol,2008,18(6):407-421. doi: 10.1002/rmv.593 [18] Stratakos A C,Linton M,Millington S,et al. A loop-mediated isothermal amplification method for rapid direct detection and differentiation of nonpathogenic and verocytotoxigenic Escherichia coli in beef and bovine faeces[J]. J Appl Microbiol,2017,122(3):817-828. doi: 10.1111/jam.13381 [19] Abdullah J,saffie N,Sjasri F A,et al. Rapid detection of salmonella typhi by loop-mediated isothermal amplification (LAMP) method[J]. Braz J Microbiol,2014,45(4):1385-1391. doi: 10.1590/S1517-83822014000400032 [20] Wu C,Zeng Y,He Y. Rapid visualization and detection of Staphylococcus aureus based on loop-mediated isothermal amplification[J]. World J Microbiol Biotechnol,2021,37(12):209. doi: 10.1007/s11274-021-03178-0 [21] Asadi R,Mollasalehi H. The mechanism and improvements to the isothermal amplification of nucleic acids,at a glance[J]. Anal Biochem,2021,62(631):114260. doi: 10.1016/j.ab.2021.114260 [22] 苑宁,张蕴哲,张海娟,等. 可视化跨越式滚环扩增技术检测食品中单核细胞增生李斯特氏菌[J]. 食品科学,2021,42(16):239-245. doi: 10.7506/spkx1002-6630-20200130-290 [23] 董晶,徐慧,郭威,等. 实时荧光跨越式滚环等温扩增结合PMA检测虾产品中的活副溶血性弧菌[J]. 食品科学,2021,42(24):289-295. doi: 10.7506/spkx1002-6630-20200927-329 [24] 李达容. 水产品中副溶血性弧菌和霍乱弧菌RAA-LFD快速检测方法的建立与应用研究[D]. 上海: 上海海洋大学,2022. [25] Wang X,Xiong E,Tian T,et al. Clustered regularly interspaced short palindromic repeats/Cas9-mediated lateral flow nucleic acid assay[J]. ACS Nano,2020,14(2):2497-2508. doi: 10.1021/acsnano.0c00022 [26] Huang M,Zhou X,Wang H,et al. Clustered regularly interspaced short palindromic repeats/Cas9 triggered isothermal amplification for site-specific nucleic acid detection[J]. Anal Chem,2018,90(3):2193-2200. doi: 10.1021/acs.analchem.7b04542 [27] Guk K,Keem J O,Hwang S G,et al. A facile,rapid and sensitive detection of MRSA using a CRISPR-mediated DNA FISH method,antibody-like dCas9/sgRNA complex[J]. Biosens Bioelectron,2017,95(9):67-71. [28] Jiang H J,Tan R,Jin M,et al. Visual detection of vibrio parahaemolyticus using combined CRISPR/Cas12a and recombinase polymerase amplification[J]. Biomed Environ Sci,2022,35(6):518-527. [29] Li F,Ye Q,Chen M,et al. Cas12aFDet: A CRISPR/Cas12a-based fluorescence platform for sensitive and specific detection of Listeria monocytogenes serotype 4c[J]. Anal Chim Acta,2021,75(1151):338248. [30] Shin H H,Hwang B H,Cha H J. Multiplex 16S rRNA-derived geno-biochip for detection of 16 bacterial pathogens from contaminated foods[J]. Biotechnol J,2016,11(11):1405-1414. doi: 10.1002/biot.201600043 [31] Sarengaowa,Hu W,Feng K,et al. An in situ-synthesized gene chip for the detection of food-borne pathogens on fresh-cut cantaloupe and lettuce[J]. Front Microbiol,2019,10(2):3089. [32] Pang B,Zhao C,Li L,et al. Development of a low-cost paper-based ELISA method for rapid Escherichia coli O157: H7 detection[J]. Anal Biochem,2018,59(542):58-62. [33] Lv X,Huang Y,Liu D,et al. Multicolor and ultrasensitive enzyme-linked immunosorbent assay based on the fluorescence hybrid chain reaction for simultaneous detection of pathogens[J]. J Agric Food Chem,2019,67(33):9390-9398. doi: 10.1021/acs.jafc.9b03414 [34] Niu K,Zheng X,Huang C,et al. A colloidal gold nanoparticle-based immunochromatographic test strip for rapid and convenient detection of Staphylococcus aureus[J]. J Nanosci Nanotechnol,2014,14(7):5151-5156. doi: 10.1166/jnn.2014.8703 [35] Zhou J,Zhang C,Zhang X,et al. Immunomagnetic separation-based nanogold enhanced surface plasmon resonance and colloidal gold test strips for rapid detection of Vibrio parahaemolyticus[J]. Arch Microbiol,2020,202(5):1025-1033. doi: 10.1007/s00203-020-01808-z [36] 王毳,闫磊,曾庆祝. 沙门氏菌的检测技术与方法[J]. 现代食品科技,2007,23(5):82-85,75. [37] Ramarao N,Tran S L,Marin M,et al. Advanced methods for detection of bacillus cereus and its pathogenic factors[J]. Sensors (Basel),2020,20(9):2667. doi: 10.3390/s20092667 [38] Xu Z,Wang J,Jia Z,et al. A microfluidic chip-based multivalent DNA walker amplification biosensor for the simultaneous detection of multiple food-borne pathogens[J]. Analyst,2023,148(5):1093-1101. doi: 10.1039/D2AN01941H -





 下载:
下载: