Molecular Mechanism of Finasteride Inhibition of Epithelial-to-Mesenchymal Transition in Bladder Cancer Cells under Hypoxia-Inducible Factor 1-alpha/Snail Family Transcriptional Repressor 1 Pathway
-
摘要:
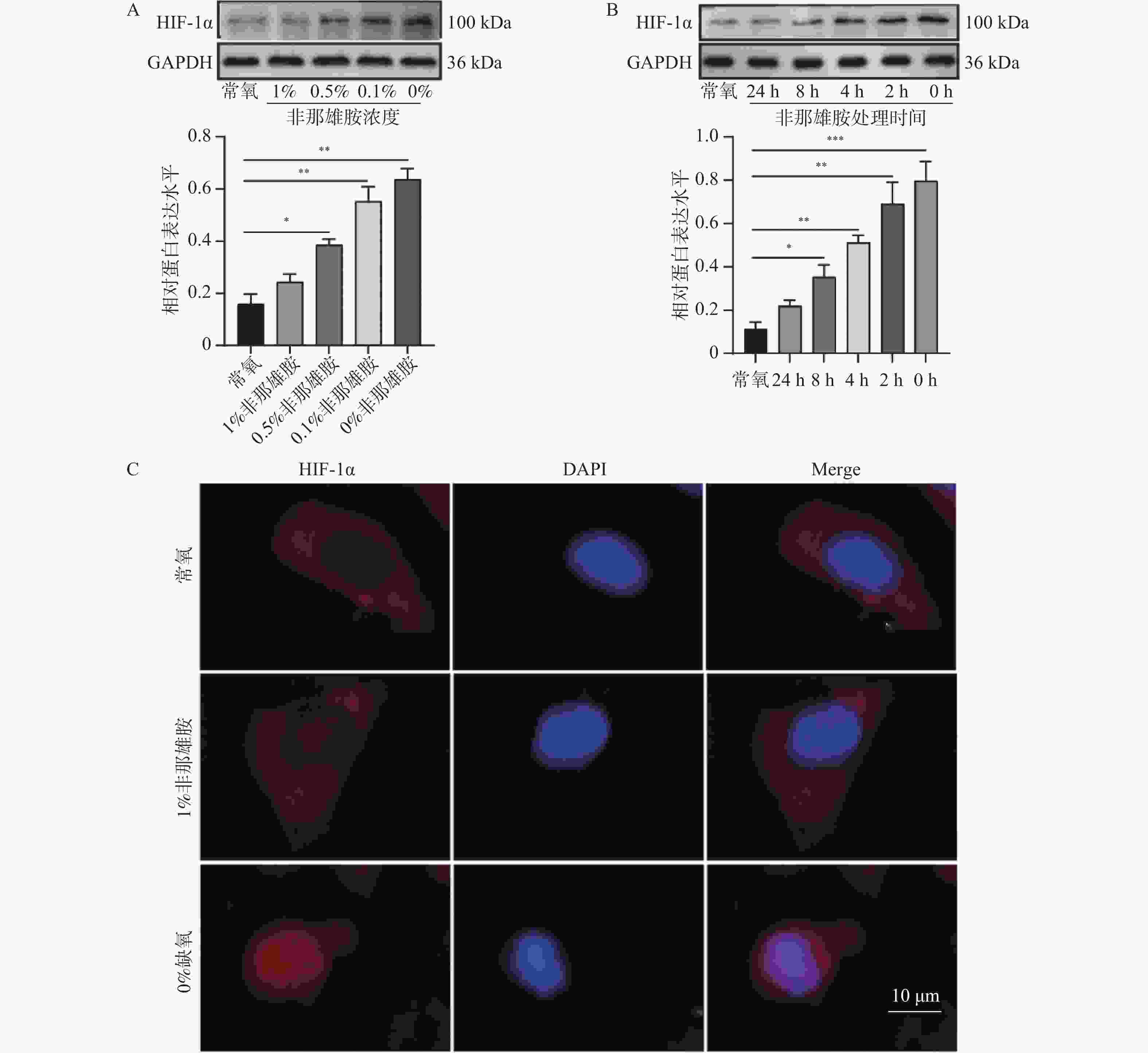
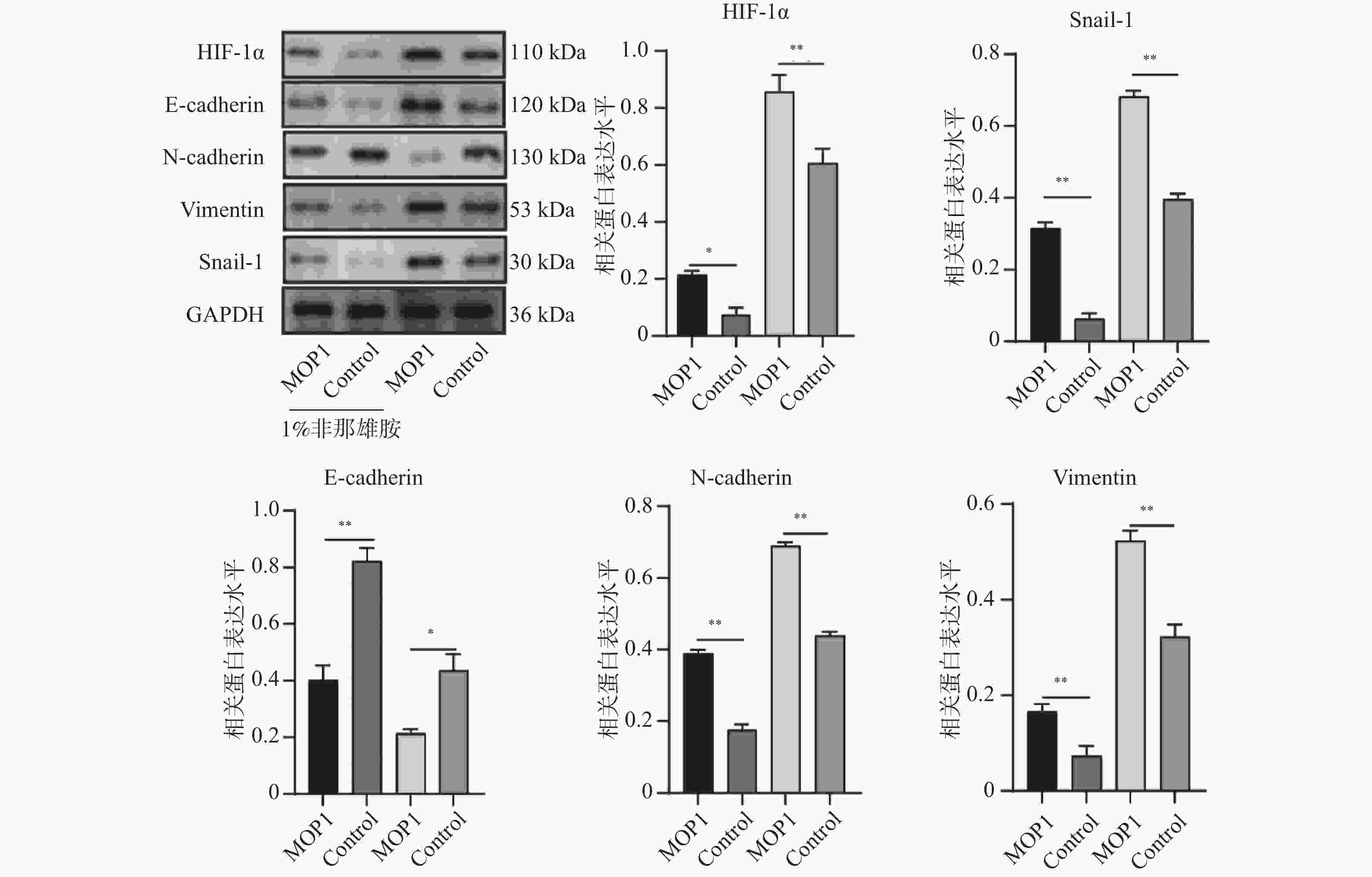
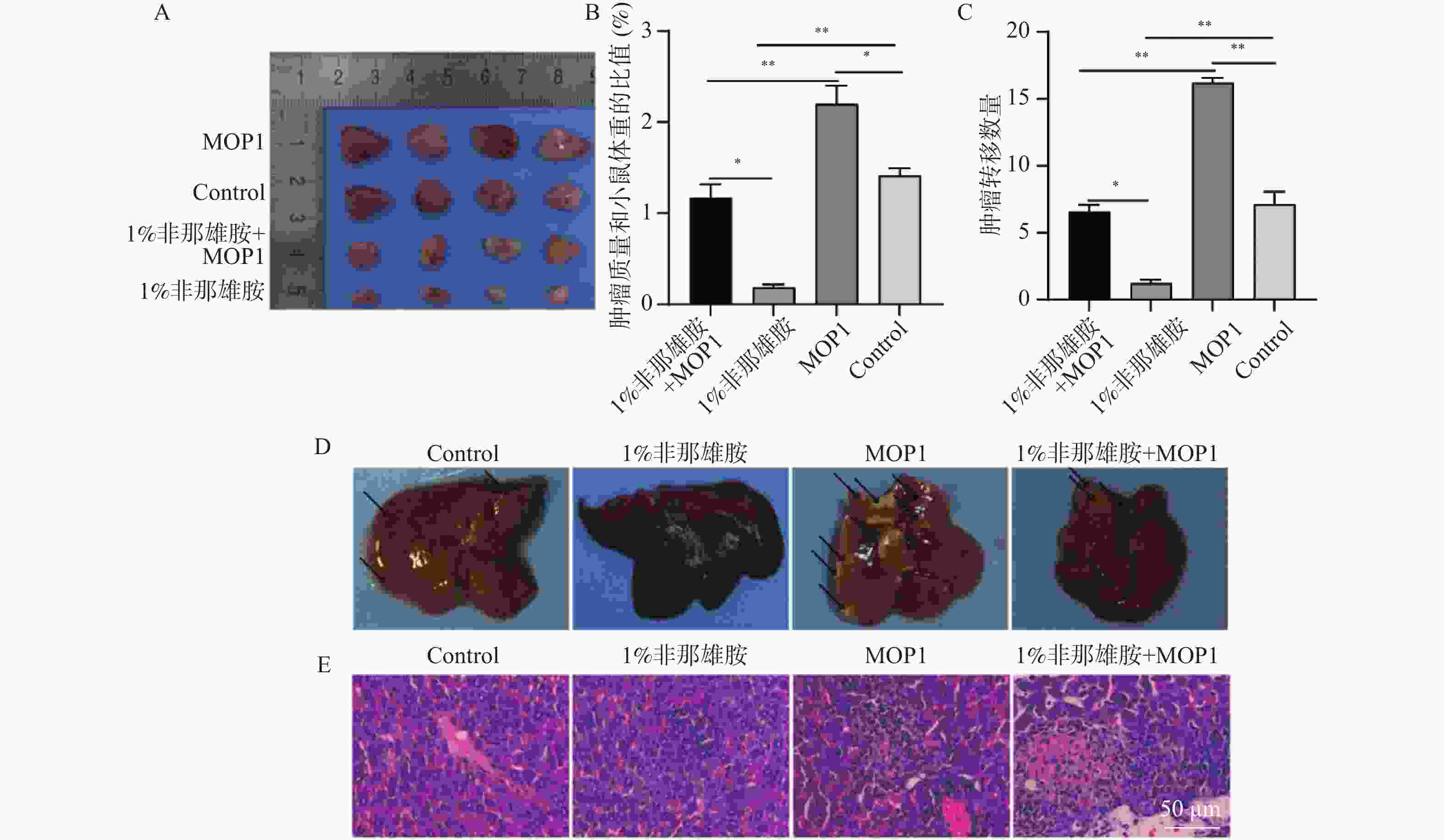
目的 探讨非那雄胺对膀胱尿路上皮癌中上皮间充质转化的影响及其机制。 方法 采用不同浓度的非那雄胺(0.1%、0.5%、1%)处理人膀胱癌T24细胞24 h,设置未处理的T24细胞为对照组。部分细胞于1% O2条件下进行缺氧处理以模拟低氧微环境。通过免疫荧光检测细胞增殖标志物Ki-67,Annexin V-FITC/PI双染流式细胞术分析细胞凋亡率,划痕实验评估细胞迁移能力,Western blot检测HIF-1α、E-cadherin、N-cadherin、Vimentin等EMT相关蛋白的表达。为进一步验证HIF-1α通路在非那雄胺抗肿瘤作用中的作用,构建HIF-1α过表达慢病毒载体(MOP-1)并转染T24细胞。动物实验中,构建T24荷瘤裸鼠模型,将小鼠随机分为对照组、MOP-1组、1%非那雄胺组和1%非那雄胺+MOP-1组,每组4只。连续灌胃给药4周后,观察肿瘤生长、转移及肝周淋巴结变化,并进行H&E染色与组织学分析。 结果 非那雄胺以浓度依赖的方式影响T24细胞的增殖、凋亡以及上皮向间充质转化。和对照组相比,非那雄胺处理的T24细胞处于生长期的数目降低,迁移的数量降低,上皮标志物表达提高,间质标志物表达降低(P < 0.05)。暴露在缺氧条件下的T24细胞中HIF-1α通路标志物的表达显著增加(P < 0.05),而1%非那雄胺处理的T24细胞中检测到HIF-1α通路被抑制(P < 0.05)。1%非那雄胺HIF-1α过表达质粒共处理组的HIF-1α通路表达水平低于对照组(P < 0.05),且小鼠肿瘤质量与体重之比较低,膀胱癌向肝转移数量降低(P < 0.05)。 结论 非那雄胺通过抑制HIF-1α/Snail表达可以有效地阻断膀胱癌生长以及上皮细胞向间充质细胞转化。 -
关键词:
- 非那雄胺 /
- 膀胱癌 /
- HIF-1α/Snail /
- 上皮-间质转化
Abstract:Objective To explore the effects and mechanism of finasteride on epithelial mesenchymal transition in bladder urothelial carcinoma. Methods T24 human bladder cancer cells were treated with different concentrations of finasteride (0.1%, 0.5%, and 1%) for 24 hours, with untreated T24 cells as the control group. Some cells were subjected to hypoxic conditions (1% O2) to mimic a low-oxygen microenvironment. Cell proliferation was assessed by immunofluorescence staining for Ki-67. Apoptosis rates were evaluated using Annexin V-FITC/PI double staining followed by flow cytometry, and cell migration was analyzed via scratch wound assays. Western blot was used to detect the expression of EMT-related proteins such as HIF-1α, E-cadherin, N-cadherin, and Vimentin. To further validate the role of the HIF-1α pathway in finasteride’ s anti-tumor effects, a HIF-1α overexpression lentiviral vector (MOP-1) was constructed and transfected into T24 cells. For animal experiment, T24 tumor-bearing nude mice were randomly divided into four groups: control, MOP-1, 1% finasteride, and 1% finasteride + MOP-1 (n = 4 per group). After 4 weeks of continuous oral administration, tumor growth, metastasis, and perihepatic lymph node changes were recorded, and histological analysis was performed using H&E staining. Results Finasteride affects the proliferation, apoptosis, and epithelial-to-mesenchymal transition of T24 cells in a concentration-dependent manner. Compared to the control group, finasteride-treated T24 cells showed a reduced number of cells in the growth phase, decreased migration, increased expression of epithelial markers, and decreased expression of mesenchymal markers (P < 0.05). In T24 cells exposed to hypoxic conditions, the expression of HIF-1α pathway markers was significantly increased (P < 0.05), while 1% finasteride treatment inhibited the HIF-1α pathway (P < 0.05). The HIF-1α pathway expression level in the 1% finasteride and HIF-1α overexpression plasmid group was lower than that in the control group (P < 0.05). Additionally, the tumor mass-to-body weight ratios were lower, with the number of bladder cancer metastases to the liver decreased (P < 0.05). Conclusion Finasteride can effectively block the growth of bladder cancer and the transition of epithelial cells into mesenchymal cells by inhibiting HIF-1α/Snail expression. -
Key words:
- Finasteride /
- Bladder cancer /
- HIF-1α/Snail /
- Epithelial mesenchymal transition
-
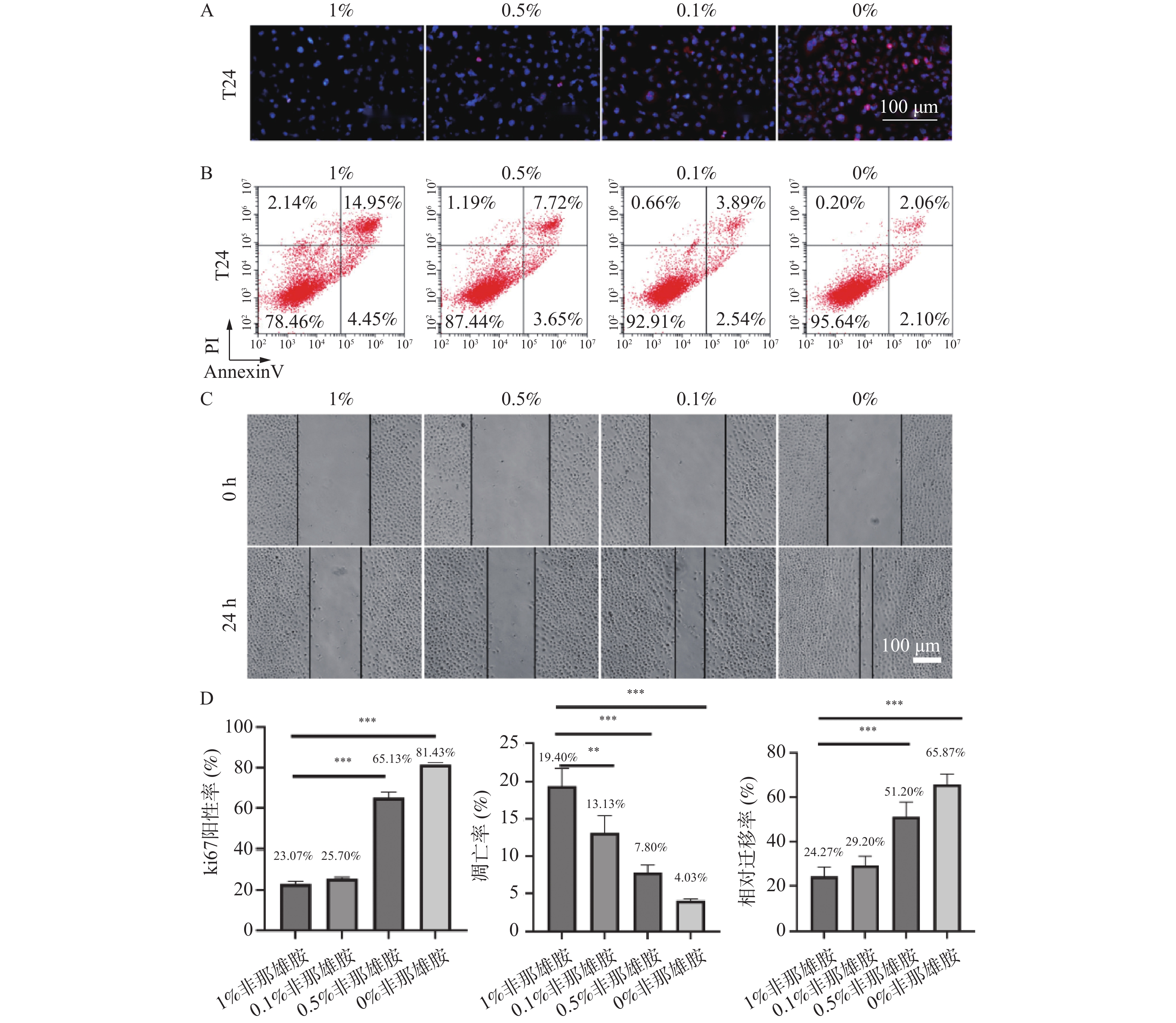
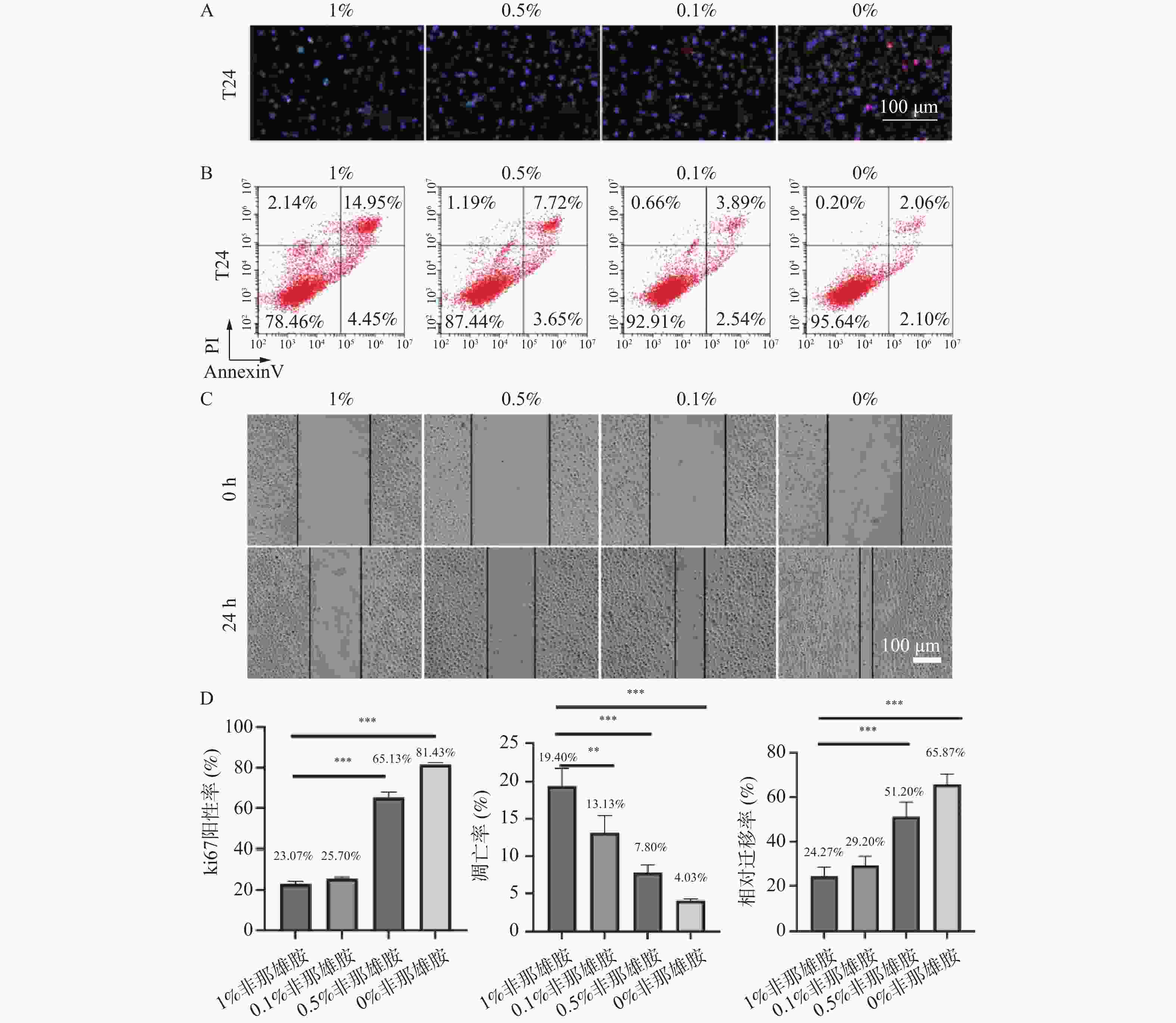
图 1 非那雄胺对膀胱上皮癌细胞增殖、凋亡及迁移的影响($\bar x \pm s $,n = 3)
A:T24细胞的增殖通过Ki-67的免疫荧光染色来评估;B:T24细胞凋亡通过Annexin V-FITC/PI染色的流式细胞术分析检测;C:24 h后T24细胞的迁移过程;D:Ki-67的免疫荧光、T24细胞凋亡、24 h后T24细胞的迁移的定量分析;**P < 0.01;***P < 0.001。
Figure 1. Effects of finasteride on proliferation, apoptosis, and migration of bladder epithelial carcinoma cells ($\bar x \pm s $,n = 3)
-
[1] Dobruch J, Oszczudłowski M. Bladder cancer: Current challenges and future directions[J]. Medicina (Kaunas), 2021, 57(8): 749. doi: 10.3390/medicina57080749 [2] Lenis A T, Lec P M, Chamie K, et al. Bladder cancer[J]. Jama, 2020, 324(19): 1980-1991. doi: 10.1001/jama.2020.17598 [3] Richters A, Aben K K H, Kiemeney L A L M. The global burden of urinary bladder cancer: An update[J]. World J Urol, 2020, 38(8): 1895-1904. doi: 10.1007/s00345-019-02984-4 [4] 陈健康, 彭道荣, 陈慧昱, 等. 尿液膀胱肿瘤抗原水平检测在膀胱癌诊断中的意义[J]. 现代检验医学杂志, 2019, 34(6): 135-137. doi: 10.3969/j.issn.1671-7414.2019.06.034 [5] 陈剑柏. 保留膀胱综合治疗对非转移性肌层浸润性膀胱癌预后影响[D]. 西安: 中国人民解放军空军军医大学, 2024. [6] 龙恭伟, 王东文, 叶章群. 肌层浸润性膀胱癌保膀胱治疗的现状与展望[J]. 现代泌尿生殖肿瘤杂志, 2023, 15(3): 129-132. doi: 10.3870/j.issn.1674-4624.2023.03.001 [7] Chislett B, Chen D, Perera M L, et al. 5-alpha reductase inhibitors use in prostatic disease and beyond[J]. Transl Androl Urol., 2023, 12(3): 487-496. [8] Crerar H, Scott Solomon E, Bodkin-Clarke C, et al. Regulation of NGF signaling by an axonal untranslated mRNA[J]. Neuron, 2019, 102(3): 553-563.e8. [9] Baumgartner B G, Orpinell M, Duran J , et al. Identification of a novel modulator of thyroid hormone receptor-mediated action[J]. PLoS One, 2007, 2(11): e1183. [10] Juri H, Narumi Y, Panebianco V, et al. Staging of bladder cancer with multiparametric MRI[J]. Br J Radiol., 2020, 93(1112): 20200116. doi: 10.1259/bjr.20200116 [11] Rashid M, Zadeh L R, Baradaran B, et al. Up-down regulation of HIF-1α in cancer progression[J]. Gene, 2021, 798: 145796. doi: 10.1016/j.gene.2021.145796 [12] Korbecki J, Simińska D, Gąssowska-Dobrowolska M, et al. Chronic and cycling hypoxia: Drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: A review of the molecular mechanisms[J]. Int J Mol Sci, 2021, 22(19): 10701. doi: 10.3390/ijms221910701 [13] Cowman S J, Koh M Y. Revisiting the HIF switch in the tumor and its immune microenvironment[J]. Trends Cancer, 2022, 8(1): 28-42. doi: 10.1016/j.trecan.2021.10.004 [14] Hayes J D, Dinkova-Kostova A T, Tew K D. Oxidative stress in cancer[J]. Cancer Cell, 2020, 38(2): 167-197. doi: 10.1016/j.ccell.2020.06.001 [15] Lee S H, Golinska M, Griffiths J R. HIF-1-independent mechanisms regulating metabolic adaptation in hypoxic cancer cells[J]. Cells, 2021, 10(9): 2371. doi: 10.3390/cells10092371 [16] Elzakra N, Kim Y. HIF-1α metabolic pathways in human cancer[J]. Adv Exp Med Biol, 2021, 1280: 243-260. [17] Zhu Y, Tan J, Xie H, et al. HIF-1α regulates EMT via the Snail and β-catenin pathways in paraquat poisoning-induced early pulmonary fibrosis[J]. J Cell Mol Med, 2016, 20(4): 688-697. doi: 10.1111/jcmm.12769 [18] De Francesco E M, Maggiolini M, Musti A M. Crosstalk between Notch, HIF-1α and GPER in breast cancer EMT[J]. Int J Mol Sci, 2018, 19(7): E2011. doi: 10.3390/ijms19072011 [19] Li Q, Hong Y, Chen J, et al. Hypoxia-induced HIF-1α expression promotes neurogenic bladder fibrosis via EMT and pyroptosis[J]. Cells, 2022, 11(23): 3836. doi: 10.3390/cells11233836 [20] Wieczorek E, Farooqi A A, Reszka E. Androgen receptor modulation and bladder cancer prevention - a short review[J]. Med Pr, 2022, 73(2): 151-162. doi: 10.13075/mp.5893.01229 [21] Martínez-Rojo E, Berumen L C, García-Alcocer G, et al. The role of androgens and androgen receptor in human bladder cancer[J]. Biomolecules, 2021, 11(4): 594. doi: 10.3390/biom11040594 [22] Tripathi A, Gupta S. Androgen receptor in bladder cancer: A promising therapeutic target[J]. Asian J Urol, 2020, 7(3): 284-290. doi: 10.1016/j.ajur.2020.05.011 [23] La Vecchia C. Finasteride and bladder cancer[J]. Eur Urol, 2016, 69(3): 411-412. doi: 10.1016/j.eururo.2015.09.001 -





 下载:
下载: