Effects of Stem Cells on Myocardial Infarction Repair and Cardiac Electrophysiology Based on Exosomal STAT3 Protein Transport
-
摘要:
目的 探讨骨髓间充质干细胞(bone marrow derived mesenchymal stem cell,BMSCs)来源外泌体(exosome,Exo)通过传递信号转导和转录激活因子3(signal transduction and transcriptional activator 3,STAT3)蛋白在心肌梗死(myocardial infarction,MI)中的作用。 方法 建立左冠状动脉前降支结扎的MI大鼠模型,随机分为Sham组、MI组及BMSCs-Exo组;另设Exo-pcDNA、Exo-pcDNA-STAT3、Exo-si-NC、Exo-si-STAT3组,研究上调或抑制Exo中STAT3表达对心功能的影响。于术后2周和4周检测大鼠心电图(electrocardiogram,ECG)、血流动力学参数,包括左心室收缩压(left ventricular systolic pressure,LVSP)、左心室舒张末期压力(left ventricular end-diastolic pressure,LVEDP)以及左心室最大上升速度(maximum rate of pressure rise in left ventricle,+dp/dtmax)和左心室最大下降速度(maximum rate of pressure decline in left ventricle,−dp/dtmax)。采用免疫蛋白印迹法免疫印迹(western blot,WB)检测心肌组织中相关蛋白表达水平,包括磷酸化信号转导与转录激活因子3(phosphorylated signal transducer and activator of transcription 3,p-STAT3)、B细胞淋巴瘤-2蛋白(b-cell lymphoma-2,Bcl-2)、Bcl-2相关X蛋白(bcl-2-associated X protein,Bax)以及切割型半胱天冬酶-3(cleaved caspase-3)。 结果 与MI组相比,BMSCs-Exo组ST段、QRS波和QT间期显著恢复(F = 23.462,P < 0.001),心律失常发生率降低(χ2 = 7.248,P = 0.009);同时LVEDP降低、LVSP及±dp/dtmax改善(F = 19.601,P < 0.001)。在转染组中,与Exo-pcDNA组相比,Exo-pcDNA-STAT3组S波振幅升高(P < 0.05),心律失常发生率下降至37.5%;与Exo-si-NC组相比,Exo-si-STAT3组QT间期延长、T波加宽、S波振幅下降(F = 30.592,P < 0.001),心律失常率升至87.5%。心功能检测显示,Exo-pcDNA-STAT3组LVEDP降低(F = 14.937,P < 0.001),而Exo-si-STAT3组ASBP、LVSP和+dp/dtmax升高(F = 16.213,P < 0.001),提示心负荷增加。WB结果显示,Exo-pcDNA-STAT3组p-STAT3与Bcl-2蛋白表达上调,而Bax及Cleaved-caspase-3下调(F = 331.518、901.008、545.635和516.670,P < 0.001);相反,Exo-si-STAT3组呈相反趋势。 结论 BMSCs-Exo可通过传递STAT3改善MI大鼠心功能、降低心律失常并减轻心肌细胞凋亡。上调STAT3可增强外泌体的心脏保护作用,抑制STAT3则削弱该效应。 -
关键词:
- 骨髓间充质干细胞 /
- 外泌体 /
- 传递信号转导和转录激活因子3 /
- 心肌梗死 /
- 心电生理
Abstract:Objective To investigate the role of bone marrow-derived mesenchymal stem cell (BMSCs)-derived exosomes (Exo) in transferring signal transduction and transcriptional activator 3 (STAT3) protein in myocardial infarction (MI). Methods A rat MI model was established by ligation of the left anterior descending coronary artery. The animals were randomly divided into Sham, MI, and BMSCs-Exo groups. Additionally, Exo-pcDNA, Exo-pcDNA-STAT3, Exo-si-NC, and Exo-si-STAT3 groups were established to investigate the effects of upregulating or inhibiting STAT3 expression in Exo on cardiac function. At 2 and 4 weeks after surgery, rat electrocardiograms (ECG) and hemodynamic parameters were measured, including left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), maximum rate of pressure rise in left ventricle (+dp/dtmax), and maximum rate of pressure decline in left ventricle (−dp/dtmax). Western blot (WB) analysis was used to detect the expression levels of related proteins in myocardial tissue, including phosphorylated signal transducer and activator of transcription 3 (p-STAT3), B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax), and cleaved caspase-3 (Cleaved-caspase-3). Results Compared with the MI group, the BMSCs-Exo group showed significant recovery of ST segment, QRS wave, and QT interval (F = 23.462, P < 0.001), and decreased arrhythmia incidence (χ2 = 7.248, P = 0.009). Simultaneously, LVEDP decreased, and LVSP and ±dp/dtmax improved (F = 19.601, P < 0.001). In the transfection groups, compared with the Exo-pcDNA group, the Exo-pcDNA-STAT3 group showed elevated S wave amplitude (P < 0.05) and reduced arrhythmia incidence to 37.5%. Compared with the Exo-si-NC group, the Exo-si-STAT3 group showed prolonged QT interval, widened T wave, and decreased S wave amplitude (F = 30.592, P < 0.001), with arrhythmia rate increased to 87.5%. Cardiac function assessment showed that the Exo-pcDNA-STAT3 group had decreased LVEDP (F = 14.937, P < 0.001), while the Exo-si-STAT3 group showed elevated ASBP, LVSP, and +dp/dtmax (F = 16.213, P < 0.001), suggesting increased cardiac workload. WB results showed that the Exo-pcDNA-STAT3 group had upregulated p-STAT3 and Bcl-2 expression, while Bax and Cleaved-caspase-3 were downregulated (F = 331.518, 901.008, 545.635, and 516.670, P < 0.001); conversely, the Exo-si-STAT3 group showed opposite trends. Conclusion BMSCs-derived exosomes improve cardiac function, reduce arrhythmias, and attenuate myocardial apoptosis after MI by delivering STAT3. Upregulation of STAT3 enhances the cardioprotective effects of exosomes, whereas STAT3 inhibition weakens these benefits. -
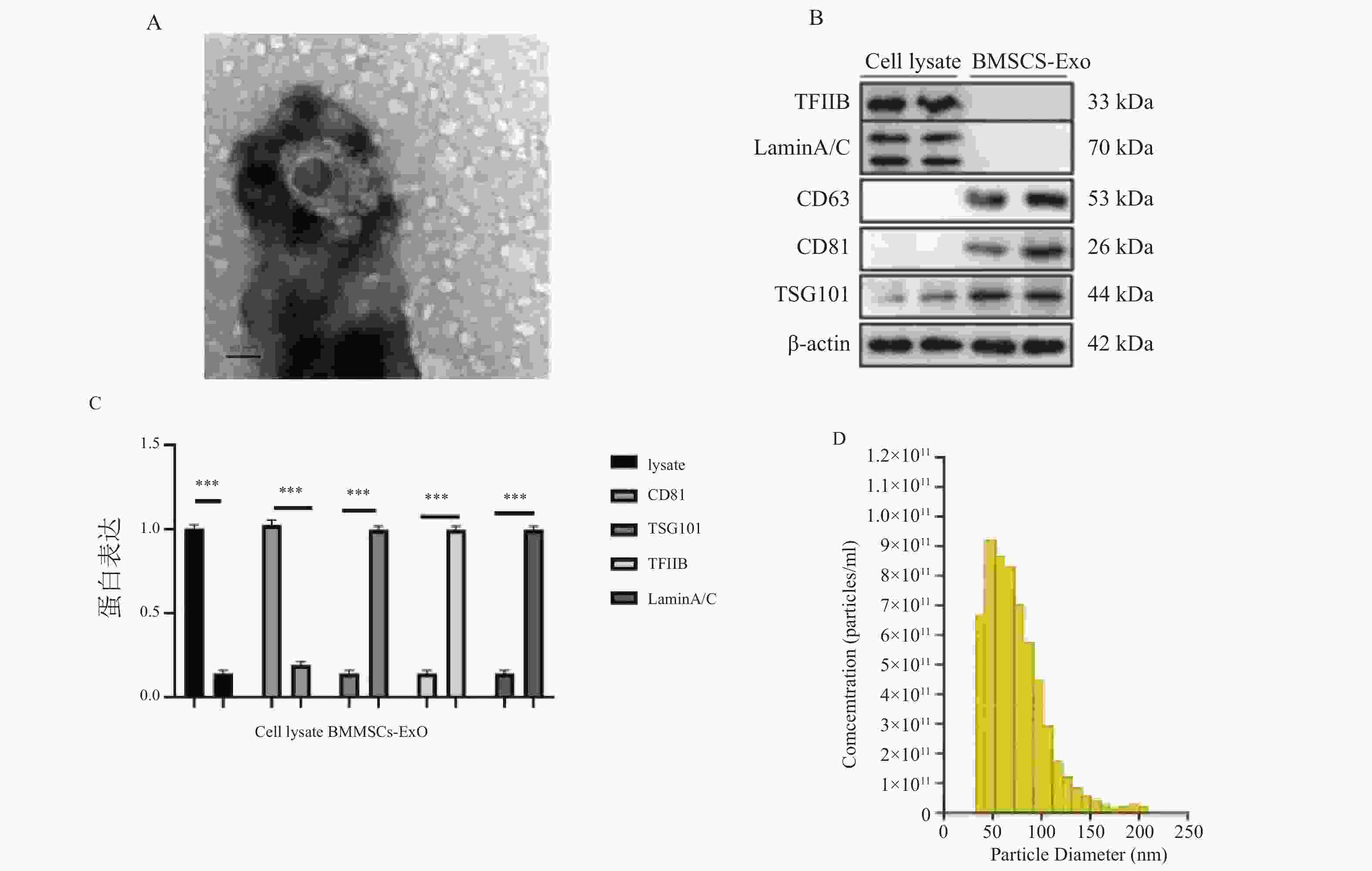
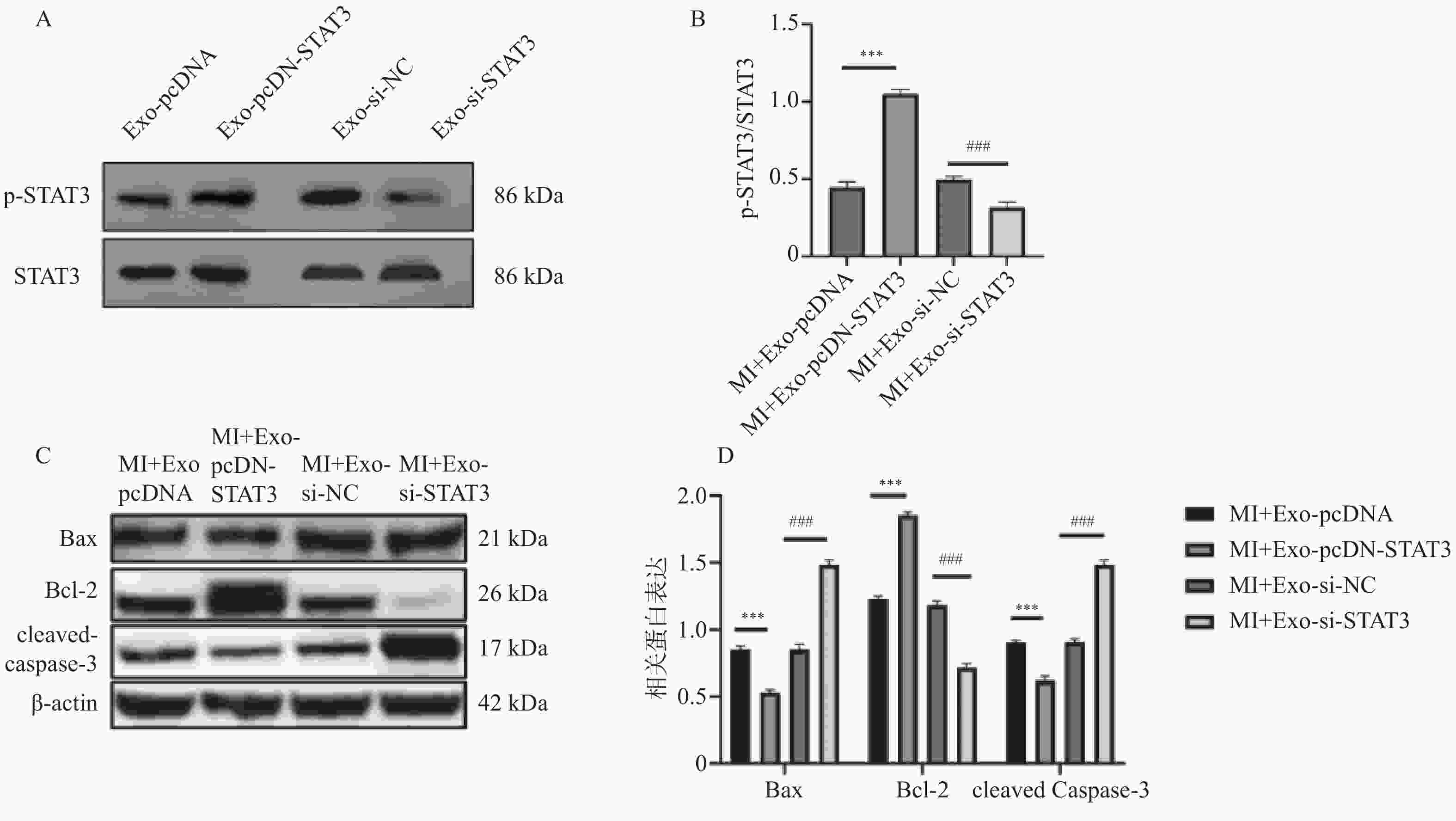
图 6 转染pcDNA-STAT3或si-STAT3对BMSCs-Exo中STAT3蛋白及凋亡相关蛋白影响($\bar x \pm s $,n = 3)
A:WB检测转染pcDNA-STAT3、si-STAT3的STAT3蛋白印迹图;B:WB检测转染pcDNA-STAT3、si-STAT3的STAT3定量分析图;C:WB检测转染pcDNA-STAT3、si-STAT3的凋亡相关蛋白蛋白印迹图;D:WB检测转染pcDNA-STAT3、si-STAT3的凋亡相关蛋白定量分析图。
Figure 6. Effect of transfecting pcDNA-STAT3 or si-STAT3 on STAT3 protein and apoptosis-related proteins in BMSCs-Exo ($\bar x \pm s $,n = 3)
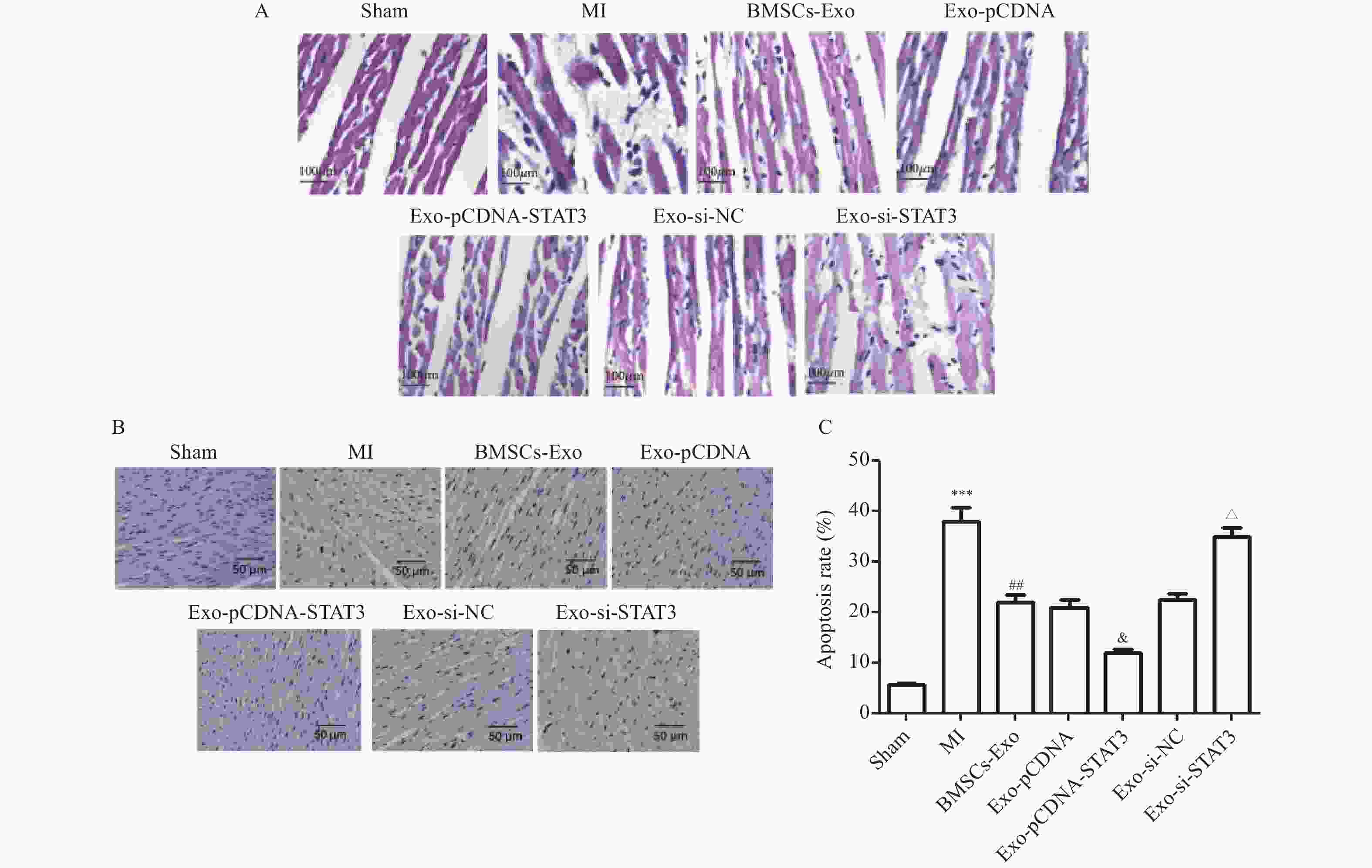
图 7 Exo-STAT3减轻MI大鼠心脏病理改变($\bar x \pm s $,n = 4)
A:各组HE染色代表性图像(×100,比例尺=100 μm);B~C:各组TUNEL染色代表性图像(×200,比例尺=50 μm);与Sham组相比,***P < 0.001;与MI组相比,##P < 0.01;与Exo-pcDNA组相比,&P < 0.05;与Exo-si-NC组相比,△P < 0.05。
Figure 7. Exo-STAT3 alleviated the cardiac physiology changes in MI rats ($\bar x \pm s $,n = 4)
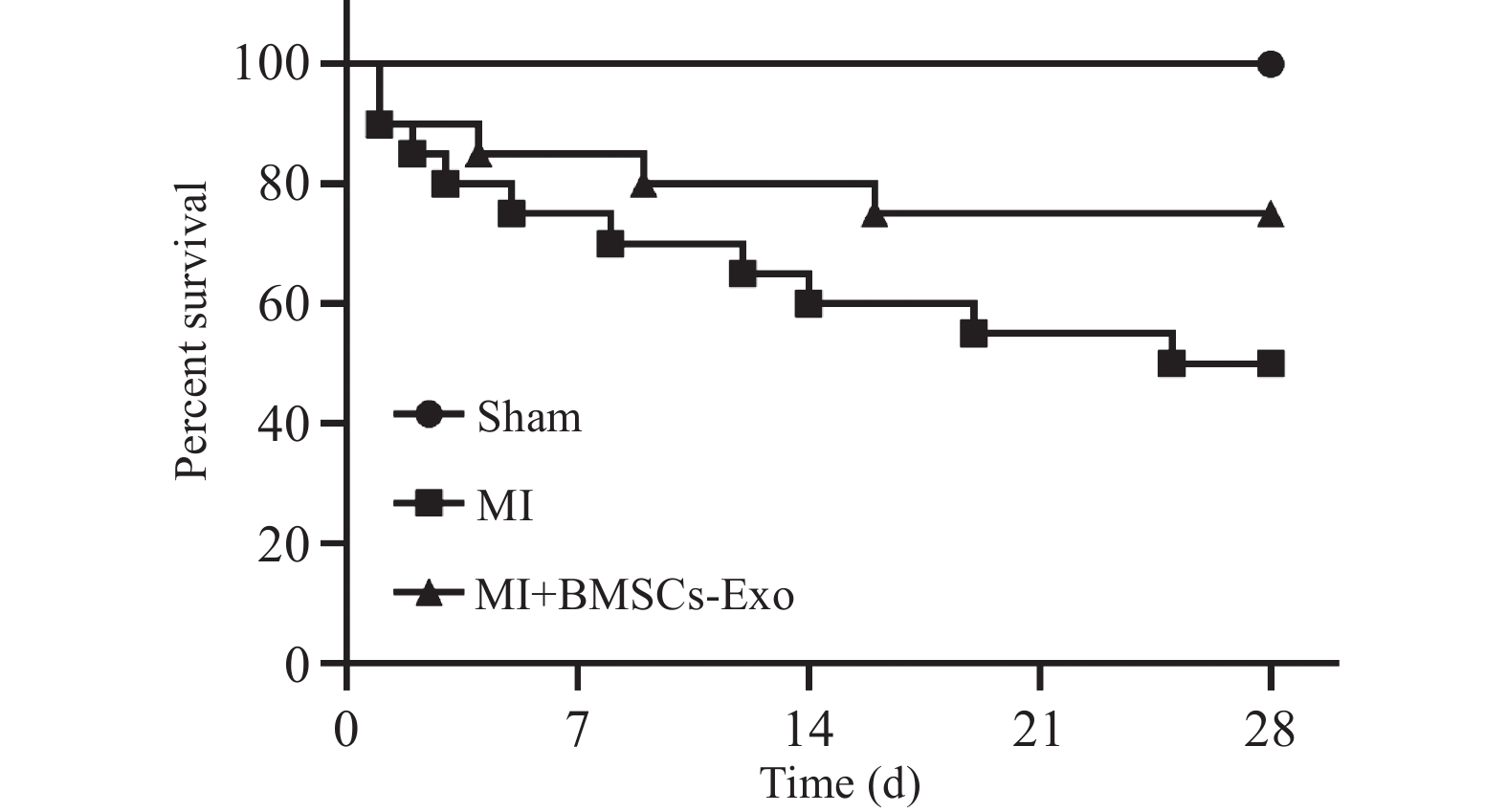
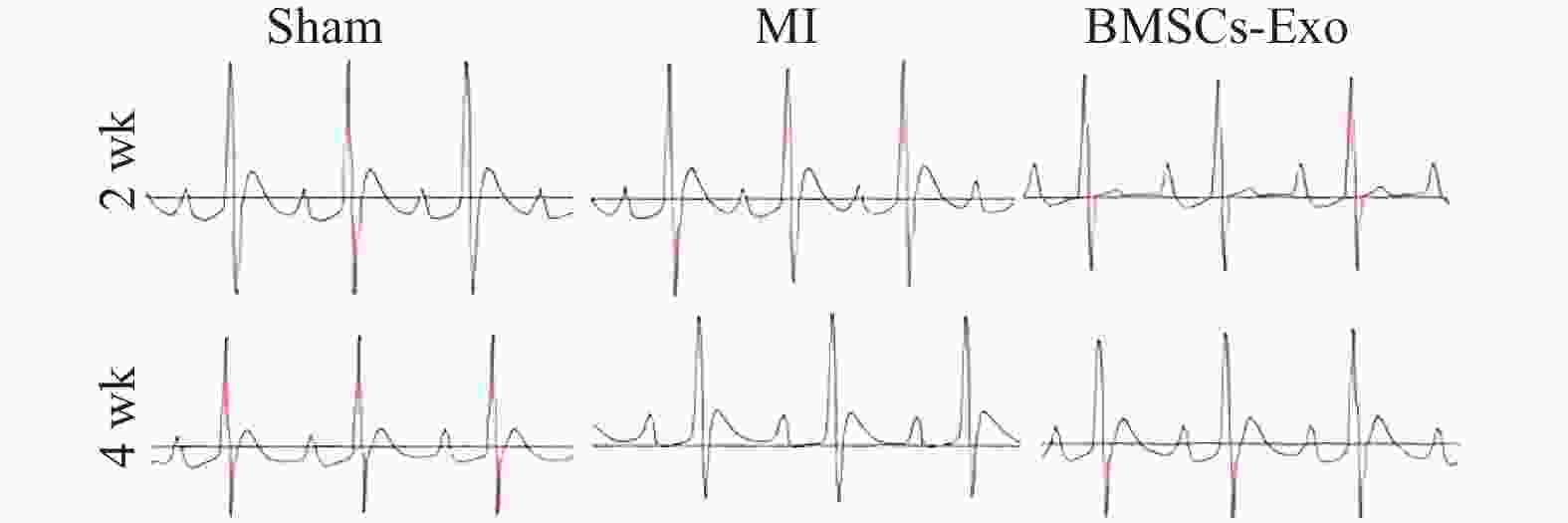
表 1 BMSCs-Exo对MI大鼠ECG的影响[($\bar x \pm s $)/n(%),n = 8)]
Table 1. Effects of BMSCs-Exo on ECG in MI rats [($\bar x \pm s $)/n(%),n = 8)]
组别 时间 P 波振幅
(mV)P 波时限
(ms)T 波时限
(ms)QT 间期
(ms)PR 间期
(ms)S 波振幅
(mV)心律失常
发生率(%)Sham 2周 0.11 ± 0.01 20.22 ± 0.62 27.56 ± 1.47 50.33 ± 1.33 45.33 ± 1.22 −0.29 ± 0.02 0(0.0) MI 0.10 ± 0.01* 20.88 ± 0.93 39.25 ± 1.50*** 60.88 ± 1.44*** 53.00 ± 2.85* −0.15 ± 0.03* 18(90.0)* BMSCs-Exo 0.12 ± 0.01# 17.25 ± 0.80 21.13 ± 2.58### 40.63 ± 3.76### 45.43 ± 1.54# −0.25 ± 0.03# 15(75.0)# F 5.241 2.126 132.567 108.324 16.237 22.415 8.754 P 0.018* 0.147 <0.001*** <0.001*** <0.001*** <0.001*** 0.005** Sham 4周 0.11 ± 0.01 17.45 ± 1.06 29.22 ± 1.61 53.56 ± 1.97 44.45 ± 1.47 −0.43 ± 0.07 0(0.0) MI 0.08 ± 0.01* 26.40 ± 1.45*** 37.38 ± 3.96 60.22 ± 3.70* 55.40 ± 0.82*** −0.16 ± 0.03** 14(70.0)* BMSCs-Exo 0.13 ± 0.01## 18.09 ± 0.97## 22.30 ± 2.37# 51.00 ± 3.54## 45.73 ± 2.14## −0.34 ± 0.06## 12(60.0)# F 10.483 18.562 65.204 44.087 32.645 38.295 9.128 P 0.003** <0.001*** <0.001*** <0.001*** <0.001*** <0.001*** 0.004** 与假手术组比较,*P < 0.05;**P < 0.01;***P < 0.001;与心肌梗死(MI)组比较,#P < 0.05;##P < 0.01;###P < 0.001。 表 2 BMSCs-Exo对MI大鼠心功能损伤的影响($\bar x \pm s $,n = 8)
Table 2. Effects of BMSCs-Exo on cardiac function in MI rats($\bar x \pm s $, n = 8)
组别 时间 心率
(次/min)平均收缩压
(mmHg)/
主动脉收缩压
(mmHg)左心室舒张
末期压力
(mmHg)左心室收缩压
(mmHg)左心室最大
上升速率
(+dp/dtmax,
mmHg/s)左心室最大
下降速率
(−dp/dtmax,
mmHg/s)Sham 2周 403.14 ± 38.88 95.31 ± 3.76 −23.47 ± 3.79 119.82 ± 3.06 3972.84 ± 122.19− 3546.74 ± 220.85MI 450.00 ± 32.07 109.80 ± 4.06* −8.92 ± 4.57* 126.38 ± 4.20 4572.79 ± 227.81*− 4031.96 ± 183.64BMSCs-Exo 376.86 ± 42.73 92.97 ± 3.94## −15.67 ± 8.09 109.07 ± 4.78# 3687.44 ± 228.53#− 3302.12 ± 262.12#F 4.891 24.674 13.554 8.242 16.733 14.92 P 0.023* <0.001*** <0.001*** 0.004** <0.001*** <0.001*** Sham 4周 372.64 ± 12.31 91.64 ± 1.64 −27.43 ± 1.79 113.58 ± 3.46 3749.03 ± 212.07− 3390.87 ± 201.06MI 428.00 ± 50.08 110.92 ± 4.63** −9.21 ± 4.25*** 135.18 ± 4.73** 4541.46 ± 308.14*− 4292.16 ± 757.17*BMSCs-Exo 399.50 ± 18.09 90.34 ± 2.57### −20.65 ± 1.90 107.83 ± 3.67## 3402.62 ± 130.43##− 2983.99 ± 139.16##F 5.546 42.38 29.761 22.424 32.295 28.741 P 0.019* <0.001*** <0.001*** <0.001*** <0.001*** <0.001*** 与假手术组比较,*P < 0.05;**P < 0.01;***P < 0.001;与MI组比较,#P < 0.05;##P < 0.01;###P < 0.001。 表 3 上调或抑制BMSCs-Exo中STAT3表达对MI大鼠ECG的影响[($\bar x \pm s $)/n(%),n = 8)]
Table 3. Effects of overexpression or suppression of STAT3 on ECG in MI rats [($\bar x \pm s $)/n(%),n = 8)]
组别 时间 P 波振幅
(mV)P 波时限
(ms)T 波时限
(ms)QT 间期
(ms)PR 间期
(ms)S 波振幅
(mV)心律失常
发生率(%)Exo-pcDNA 2周 0.12 ± 0.02 18.88 ± 0.77 20.63 ± 8.73 42.14 ± 2.41 45.38 ± 1.63 −0.25 ± 0.08 15(75.0) Exo-pcDNA-STAT3 0.12 ± 0.01 19.33 ± 0.72 23.50 ± 2.69 47.60 ± 3.34 45.10 ± 1.62 −0.31 ± 0.04* 9(45.0)* Exo-si-NC 0.12 ± 0.01 17.55 ± 0.80 21.13 ± 2.58 41.63 ± 3.57 45.43 ± 1.54 −0.25 ± 0.03 14(70.0) Exo-si-STAT3 0.10 ± 0.01# 17.7 ± 0.95 31.13 ± 2.58# 55.63 ± 4.45# 49.43 ± 1.54# −0.20 ± 0.03# 16(80.0)# F 4.227 1.733 22.843 30.592 18.416 6.981 7.248 P 0.028* 0.195 <0.001*** <0.001*** <0.001*** 0.011*** 0.009** Exo-pcDNA 4周 0.10 ± 0.01 19.33 ± 0.72 29.50 ± 2.69 51.60 ± 3.34 45.10 ± 1.62 −0.34 ± 0.04 11(55.0) Exo-pcDNA-STAT3 0.10 ± 0.01 18.90 ± 1.18 27.89 ± 1.21 48.44 ± 1.32 46.44 ± 1.25 −0.40 ± 0.04* 5(25.0)* Exo-si-NC 0.10 ± 0.01 18.55 ± 0.65 27.45 ± 2.71 50.40 ± 2.46 45.20 ± 1.31 −0.33 ± 0.04 10(50.0) Exo-si-STAT3 0.07 ± 0.01# 19.09 ± 0.97 35.30 ± 2.37# 56.00 ± 3.54# 50.22 ± 2.47# −0.23 ± 0.04# 13(65.0)# F 5.902 1.284 19.626 21.341 16.853 8.115 6.507 P 0.017* 0.292 <0.001*** <0.001*** <0.001*** 0.01* 0.015* 与Exo-pcDNA组相比,*P < 0.05;**P < 0.01;***P < 0.001;与Exo-si-NC组相比,#P < 0.05。 表 4 上调或抑制BMSCs-Exo中STAT3表达对MI大鼠心功能损伤的影响($\bar x \pm s $,n = 8)
Table 4. Effects of overexpression or suppression of STAT3 on cardiac function in MI rats ($\bar x \pm s $,n = 8)
组别 时间 心率
(次/min)平均收缩压
(mmHg)/
主动脉收缩压
(mmHg)左心室舒张
末期压力(mmHg)左心室收缩压
(mmHg)左心室最大
上升速率
(+dp/dtmax,
mmHg/s)左心室最大
下降速率
(−dp/dtmax,
mmHg/s)Exo-pcDNA 2周 400.38 ± 23.00 93.83 ± 3.86 −14.69 ± 2.89 109.51 ± 2.83 3646.49 ± 198.24− 3312.45 ± 329.80#Exo-pcDNA-STAT3 381.63 ± 33.58 96.67 ± 2.92 −20.42 ± 3.88* 105.89 ± 5.34 3849.67 ± 237.23− 3317.31 ± 183.80Exo-si-NC 386.40 ± 41.21 92.41 ± 2.85 −15.53 ± 2.37 108.28 ± 4.90 3602.17 ± 235.64− 3305.10 ± 383.84Exo-si-STAT3 406.75 ± 34.18 100.34 ± 3.45# −10.13 ± 4.28# 120.38 ± 4.20# 4172.79 ± 227.81#− 3884.09 ± 255.92#F 3.562 11.872 14.937 16.213 19.601 18.447 P 0.048* 0.002*** <0.001*** <0.001*** <0.001*** <0.001*** Exo-pcDNA 4周 389.33 ± 10.04 92.42 ± 4.32 −20.98 ± 1.98 109.06 ± 4.97 3476.77 ± 369.09− 3070.13 ± 210.55Exo-pcDNA-STAT3 387.30 ± 18.66 93.51 ± 4.83 −27.18 ± 2.69* 107.76 ± 5.11 3679.16 ± 402.39− 3294.09 ± 289.27Exo-si-NC 385.82 ± 28.82 93.23 ± 2.72 −20.84 ± 1.77 108.82 ± 5.65 3437.62 ± 203.45− 3117.43 ± 228.77Exo-si-STAT3 402.50 ± 17.21 101.91 ± 4.48# −12.26 ± 7.39# 117.51 ± 6.02# 4045.35 ± 416.49#− 3970.24 ± 244.74#F 4.615 10.763 15.081 13.442 14.926 17.772 P 0.031* 0.003** <0.001*** 0.001*** <0.001*** <0.001*** 与Exo-pcDNA组相比,*P < 0.05;**P < 0.01;***P < 0.001;与Exo-si-NC组相比,#P < 0.05。 -
[1] Vogel B, Claessen B E, Arnold S V, et al. ST-segment elevation myocardial infarction[J]. Nat Rev Dis Primers, 2019, 5(1): 39. doi: 10.1038/s41572-019-0090-3 [2] 李思懿, 公威, 赵冠棋, 等. 急性心肌梗死后心脏破裂临床预测因素研究现状[J]. 中华急诊医学杂志, 2020, 29(3): 437-442. [3] Omar A M, Meleis A E, Arfa S A, et al. Comparative study of the therapeutic potential of mesenchymal stem cells derived from adipose tissue and bone marrow on acute myocardial infarction model[J]. Oman Med J, 2019, 34(6): 534-543. doi: 10.5001/omj.2019.97 [4] Pan W, Zhu Y, Meng X, et al. Immunomodulation by exosomes in myocardial infarction[J]. J Cardiovasc Transl Res, 2019, 12(1): 28-36. doi: 10.1007/s12265-018-9836-7 [5] 王佳, 林雪容, 高恒波, 等. miR-29a靶向信号转导及转录激活因子3调控炎症反应在脂多糖诱导大鼠肾小管上皮细胞损伤中的保护作用[J]. 实用医学杂志, 2020, 36(21): 2906-2910+2915. doi: 10.3969/j.issn.1006-5725.2020.21.004 [6] 向杰, 刘明鑫, 黄从新. STAT3信号通路在心房颤动中的机制研究[J]. 心血管病学进展, 2020, 41(1): 22-26. doi: 10.16806/j.cnki.issn.1004-3934.2020.01.007 [7] Zhang Q, Liu R X, Chan K W, et al. Exosomal transfer of p-STAT3 promotes acquired 5-FU resistance in colorectal cancer cells[J]. J Exp Clin Cancer Res, 2019, 38(1): 320. doi: 10.1186/s13046-019-1314-9 [8] 饶兰兰, 马添翼. 前列腺素E1通过抑制内质网应激保护心肌梗死后大鼠的心脏[J]. 中国病理生理杂志, 2020, 36(6): 1027-1033. doi: 10.3969/j.issn.1000-4718.2020.06.010 [9] 贺继刚, 谢巧丽, 王梓豪, 等. 过表达心肌细胞转录因子骨髓间充质干细胞分泌外泌体抗心肌细胞凋亡的分子调控机制[J]. 医学研究生学报, 2019, 32(9): 910-914. doi: 10.16571/j.cnki.1008-8199.2019.09.003 [10] Xiong L, Liu Y, Zhou M, et al. Targeted ablation of cardiac sympathetic neurons attenuates adverse postinfarction remodelling and left ventricular dysfunction[J]. Exp Physiol, 2018, 103(9): 1221-1229. doi: 10.1113/EP086928 [11] Li J, Cai S X, He Q, et al. Intravenous miR-144 reduces left ventricular remodeling after myocardial infarction[J]. Basic Res Cardiol, 2018, 113(5): 36. doi: 10.1007/s00395-018-0694-x [12] Inoue M, Noda R, Yamaguchi S, et al. Specific factors to predict large-vessel occlusion in acute stroke patients[J]. J Stroke Cerebrovasc Dis, 2018, 27(4): 886-891. doi: 10.1016/j.jstrokecerebrovasdis.2017.10.021 [13] Guo R, Morimatsu M, Feng T, et al. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction[J]. Stem Cell Res Ther, 2020, 11(1): 19. doi: 10.1186/s13287-019-1536-y [14] Zhang J, Zhang X. Ischaemic preconditioning-induced serum exosomes protect against myocardial ischaemia/reperfusion injury in rats by activating the PI3K/AKT signalling pathway[J]. Cell Biochem Funct, 2021, 39(2): 287-295. doi: 10.1002/cbf.3578 [15] 罗超, 黄磊, 吴平平, 等. 间充质干细胞外泌体中的RNA与蛋白质[J]. 中国细胞生物学学报, 2020, 42(3): 519-524. doi: 10.11844/cjcb.2020.03.0019 [16] 黄发, 吕嘉贤, 许静红, 等. 外泌体介导miR-519c-5p增强脂多糖诱导的血管内皮细胞凋亡作用[J]. 实用医学杂志, 2020, 36(6): 722-728. doi: 10.3969/j.issn.1006-5725.2020.06.004 [17] Zhang W Y, Zhang Q L, Xu M J. Effects of propofol on myocardial ischemia reperfusion injury through inhibiting the JAK/STAT pathway[J]. Eur Rev Med Pharmacol Sci, 2019, 23(14): 6339-6345. [18] Pipicz M, Demján V, Sárközy M, et al. Effects of cardiovascular risk factors on cardiac STAT3[J]. Int J Mol Sci, 2018, 19(11): 3572. doi: 10.3390/ijms19113572 -





 下载:
下载: