Effectiveness of Low Intensity Focused Ultrasound Stimulation of Nucleus Accumbens in Mice
-
摘要:
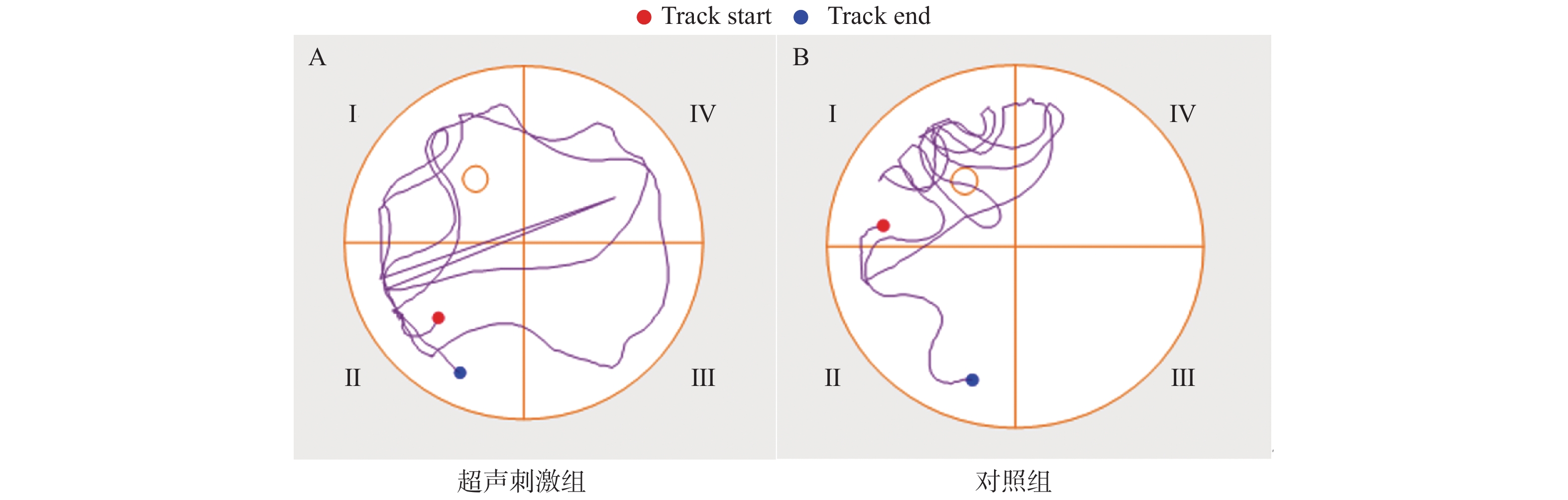
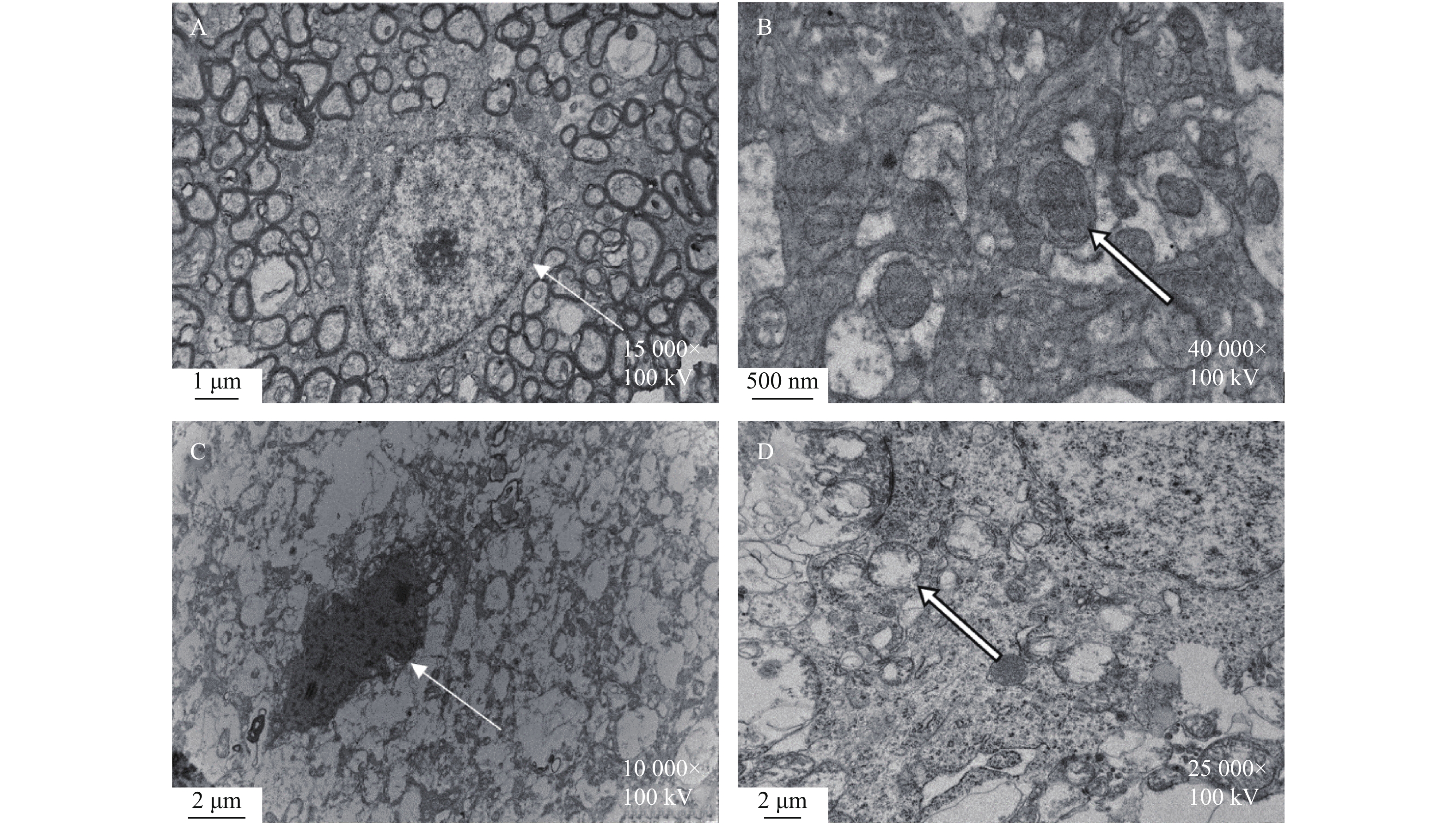
目的 使用低强度聚焦超声对正常小鼠的伏隔核进行超声刺激,从行为学、病理改变以及神经生物化学方面,探讨超声刺激进行神经调控的有效性。 方法 100只C57BL/6小鼠随机分为实验组和对照组各50只,实验组给予经颅低强度聚焦超声对伏隔核进行超声刺激,每天2次,每次10 min,连续刺激7 d;对照组给予假刺激。干预结束后,2个大组内又随机分为行为学评估30只(分别进行强迫游泳、旷场和水迷宫实验)、电镜检测10只和神经递质检测10只,立刻进行相关检测。强迫游泳实验评估小鼠的抑郁程度,旷场实验评估小鼠的焦虑程度,水迷宫实验评估小鼠的学习记忆能力。透射电镜观察小鼠伏隔核神经元超微结构的改变,ELISA法测定小鼠NAc中单胺类神经递质(去甲肾上腺素、多巴胺、5-羟色胺)的含量。 结果 (1)行为学评价结果:与空白对照组比较,超声刺激组在强迫游泳实验中不动时间明显延长(P < 0.001),在旷场中行进总距离、行进平均速度、中央区活动距离、中央区停留时间和中央区穿越次数均显著减少( P < 0.05, P < 0.01),在水迷宫实验中逃避潜伏时间明显延长,穿越平台次数明显减少,在目标象限停留时间明显缩短( P < 0.05, P < 0.001);(2)电镜结果:对照组NAc神经元超微结构基本正常,超声刺激组神经元肿胀,神经纤维溶解坏死,胞浆内细胞器减少或消失,线粒体空泡化,内质网扩张;(3)神经递质测定结果:与对照组比较,超声刺激组小鼠NAc单胺类神经递质去甲肾上腺素、多巴胺、5-羟色胺的含量均显著降低( P < 0.0001)。 结论 低强度聚焦超声对小鼠可产生抑制性的神经调节作用,并造成NAc神经元形态学以及神经生物化学改变,这可能是超声神经调控的机制之一。 Abstract:Objective To investigate the effectiveness of ultrasound stimulation for neural regulation from the aspects of behavioral pathology and neurobiochemistry by using low intensity focused ultrasound to stimulate the nucleus accumbens of normal mice. Methods 100 C57BL/6 mice were randomly divided into experimental group and control group with 50 mice in each group. The control group was given sham stimulation, and the experimental group was given transcranial low-intensity focused ultrasound to stimulate the NAc, twice a day, 10 min each time, for 7 consecutive days. After the intervention, 30 mice were randomly assigned to behavioral assessment (forced swimming, open field, and water maze), 10 mice were assigned to electron microscopy (EM), and 10 mice were assigned to neurotransmitter test.The depression, anxiety and learning and memory ability of the mice were evaluated by forced swimming, open field and water maze tests.The ultrastructural changes of neurons in the NAcof mice were observed by transmission electron microscopy. The contents of monoamine neurotransmitters (norepinephrine, dopamine, 5-hydroxytryptamine) in NAc of mice were determined by ELISA. Results 1. Behavioral evaluation results: Compared with the control group, the immobile time in the ultrasonic stimulation group was significantly longer (P < 0.01), the total distance travelled, the average speed travelled, the distancetravelled of the central area, the time of the central area and the number of exits from the central area in the open field all decreased significantly ( P < 0.05, P < 0.01), In the water maze experiment, the avoidance latency time was significantly longer, the number of crossing the platform was significantly reduced, and the residence time in the target quadrant was significantly shorter ( P < 0.05, P < 0.01). 2. Electron microscopy results: The ultrastructure ofNAc neurons in the control group was basically normal. In the ultrasound stimulation group, the neurons were swelling, nerve fiber dissolution and necrosis, mitochondrial vacuolation, endoplasmic reticulum dilation, and intracytoplasmic organelles were reduced or disappeared.3. Measurement results of neurotransmitters: Compared with the control group, the contents of NAc monoamine neurotransmitters NE, DA and 5-HT were significantly decreased in the ultrasonic stimulation group ( P < 0.05). Conclusion Low intensity focused ultrasound can induce inhibitory neuroregulation in mice, and cause morphological and neurobiochemical changes in NAC neurons, which may be one of the mechanisms of ultrasound neuromodulation. -
Key words:
- Low intensity focused ultrasound /
- Neuromodulation /
- Nucleus accumbens /
- Effectiveness
-
帕金森病(Parkinson’s disease,PD)是一种中枢神经系统变性疾病,病情呈进行性发展,好发于中老年人,以肢体僵直、运动迟缓和震颤为主要特征[1]。研究表明,30.0%~86.8%[2]的PD患者存在睡眠障碍,失眠是其最常见类型,主要表现为入睡困难、睡眠维持困难、早醒、睡眠质量降低及时间减少并伴有日间功能障碍[3]。长期失眠会严重影响PD患者的认知,增加其焦虑、抑郁情绪等负性情绪,从而导致患者更严重的失眠,甚至形成恶性循环[4]。临床上对PD患者失眠的治疗包括药物治疗和非药物治疗,但药物治疗易发生不良反应,如药物依赖、日间嗜睡、认知功能损害等,且停药后易形成反跳性失眠。非药物治疗常采用物理治疗、失眠的认知行为治疗以及补充替代治疗。而2022年中国PD睡眠障碍管理专家共识指出失眠认知行为疗法(cognitive behavior therapy for insomnia,CBT-I)是非药物治疗方式中的首选方法,同时该治疗被美国睡眠医学学会作为失眠治疗的A级推荐[2]。
CBT-I是基于睡眠卫生教育、刺激控制治疗、睡眠限制、矛盾意向及放松训练等方式帮助被治疗者改善其关于睡眠的不合理信念和行为,为被治疗者提供合理的建议以及干预策略,改善其睡眠障碍的一种心理干预方法[5]。CBT-I治疗安全性、有效性相对较好,其对失眠症治疗效果已得到研究证实[6],但对PD伴失眠患者的效果有待进一步探讨。本研究对PD患者实施8周的CBT-I,探讨CBT-I对PD患者睡眠结构及睡眠质量的影响。
1. 资料与方法
1.1 研究对象
选取2021年1月至2022年3月在河北省某三甲医院住院的PD伴失眠患者73例,使用随机数字表法设置试验组和对照组。纳入标准:(1)PD的诊断符合《中国帕金森病的诊断标准(2016版)》[7];(2)失眠符合2017年中国睡眠研究会《失眠症的诊断及治疗》[8];(3)年龄45~75岁,改良Hoehn-Yahr分级≤3级者[9];(4)能够配合量表评测;(5)在入组前接受固定的抗PD药物治疗至少1个月以上;(6)患者知情同意。排除标准:(1)合并失眠以外的其他睡眠障碍诊断(如睡眠呼吸暂停)者;(2)合并精神障碍、酒精成瘾以及其他严重疾病者;(3)认知功能障碍者;(4)有帕金森叠加综合征、继发性帕金森综合征等非原发性帕金森病。剔除标准:(1)患者依从性差,连续2周未按试验要求进行治疗者;(2)病情急性加重、主动退出以及其他原因失访者。在后续随访的过程中,试验组有3名受试者脱落,对照组有4名受试者脱落,最终对完成试验的66名受试者数据进行分析,其中试验组33例,对照组33例。本研究已通过医院伦理委员会批准(20211202)。
1.2 干预方法
1.2.1 对照组
进行常规健康教育,包括向患者讲解PD以及睡眠的相关知识,告知目前的诊疗进展,鼓励患者养成健康卫生生活习惯,规律作息,合理安排饮食,进行适当运动,戒烟戒酒,遵医嘱用药以及进行常规心理护理等指导。
1.2.2 试验组
(1)组建CBT-I护理小组:组建CBT-I护理小组,成员包括护士长1名、神经内科主任医师1名及中级及以上职称专科护理人员5名。小组成员均进行过专业的CBT-I知识及技能培训,并通过相应考核。由小组成员对患者进行一对一个体化的CBT-I干预治疗。(2) CBT-I的实施:在常规健康教育的基础上,试验组每周接受一次CBT-I治疗,单次持续时间为1 h,总干预时间为8周,具体实施过程步骤如下。
第1部分:CBT-I睡眠训练部分(第1~4周):第1周,取得合作。进行自我介绍并相互熟悉,讲解CBT-I治疗的目的、规则及实施步骤流程。初步了解患者的睡眠问题,共同制定预期达到的睡眠目标。讲解睡眠日记的记录方法和时间。指导并协助完成睡眠日记的记录。第2周,进行睡眠卫生知识教育。通过睡眠日记了解患者具体睡眠情况,以“失眠的3P模型”[10] 为指导,与患者共同讨论其失眠的易感因素、发病诱因、严重程度、类型和特征,找出患者失眠的维持因素,帮助识别改正不良的睡眠习惯并为患者发放《睡眠健康管理手册》,复杂/特殊情况由小组成员共同分析讨论。第3周,讲解刺激控制治疗的内容。指导患者改变影响卧床与睡意之间相互作用的不良行为,恢复卧床原有的诱导睡眠信号功能,重建患者床/卧室和睡眠间的条件反射[11]。要求患者只有感到睡意十分强烈时再上床睡觉,若卧床超过20 min但仍无法入睡或产生挫败感,应起床离开卧室,直至再次困倦再返回卧室睡觉。并且不能在床上做与睡眠无关的任何事情,如胡思乱想、进食、看手机以及看电视等。避免日间小睡。第4周,讲解睡眠限制治疗的内容。根据睡眠日记,包括入睡潜伏期、总卧床时间、入睡后觉醒次数和时间、实际睡眠时长,计算出平均睡眠效率,帮助调整患者的总卧床时间,提高其睡眠效率。
第2部分:CBT-I放松训练部分(第5周):第5周,指导患者进行放松训练。教会患者渐进性肌肉放松训练、正念呼吸、冥想、音乐放松等多种方法,并结合患者的感受形成个体化放松训练方案,嘱患者反复练习并给予指导。引导患者在不良情绪发生时采取适宜的应对方式平复内心。
第3部分:CBT-I行为训练部分(第6~8周):第6周,进行睡眠认知重组。探讨识别患者的错误睡眠认知以及负性睡眠信念,比如对睡眠有过高的期望、将失眠的影响灾难化、所有的过错都归因于失眠等,解释不合理的睡眠信念所产生的不良影响,减少和消除患者对失眠的恐惧及焦虑,重塑正性睡眠思维模式。第7周,行为训练强化。利用训练成果强化其心理认知。找出患者尚存的不合理行为,制定个性化的睡眠行为处方,帮助其解决治疗过程中不良思维模式,建立良好的睡眠行为习惯。第8周,沟通与反馈。鼓励患者主动就治疗过程和效果进行沟通,进行有效反馈。回顾CBT-I治疗的训练内容,讨论治疗效果,肯定取得的进步并为未来制定远期计划。
1.3 收集资料
1.3.1 评估工具(1)一般情况调查问卷
内容包括年龄、性别、PD病程、Hoehn-Yahr分期、失眠病程、睡眠习惯等基本信息,由研究者自行编制。(2)匹兹堡睡眠质量指数(pittsburgh sleep quality index,PSQI)[12] 量表用于评定患者最近1个月的睡眠质量,包含19个自评条目,共7个维度,每个维度0~3分计分,累积各维度得分为PSQI总分,总分0~21分,国内一般将7分为睡眠质量好坏的临界值,分数越高说明睡眠质量越差。(3)失眠严重程度指数(insomnia severity index,ISI)[13]是筛查和衡量临床患者失眠严重程度的简便有效且可靠的工具,包括入睡困难、睡眠维持、早醒、睡眠模式的满意程度、失眠对日间功能的干扰、失眠对生活质量的影响以及失眠的情绪反应,共7个条目,每个条目均采用Likert-5级评分法进行评分,总分0~28分,分数越高,失眠的严重程度越高。(3)汉密尔顿抑郁量表(hamilton depression scale,HAMD17)[14] 是临床上评定抑郁状态时应用最为普遍的量表。总分为53分,HAMD17≤7分为无抑郁症状,HAMD17>7分且分值越高表示患者抑郁程度越重。(4)汉密尔顿焦虑量表(hamilton anxiety scale,HAMA14)[15] 用来评估患者焦虑情况,总分为56分,HAMA14<7分为无焦虑症状,HAMA14≥7分且分值越高表示患者焦虑程度越重。
1.3.2 睡眠日记
睡眠日记是一种自我管理的睡眠行为记录。包括患者记录自己晚间的睡眠情况、早上起床情况以及对自己睡眠质量的评价等内容。通过7 d的睡眠情况记录可以分析患者入睡潜伏期(sleep onset latency,SOL)、总睡眠时间(total sleep time,TST)和睡眠效率(sleep efficiency,SE)等多个因子。
1.3.3 评估时间
干预前收集患者的一般情况调查问卷,评估患者的PSQI、ISI、HAMA14、HAMD17水平。治疗第8周再次进行PSQI、ISI、HAMA14、HAMD17评估。要求患者每天记录睡眠日记,包括上床时间、起床时间、SOL、TST等条目。
1.4 统计学处理
数据采用IBM SPSS 25.0统计软件处理,对所有数据进行正态性检验,符合正态分布的计量资料采用均数、标准差进行描述,采用配对样本t检验进行组内治疗前后数据比较,采用两独立样本t检验进行组间比较;非正态分布采用M(P25,P75)表示。计数资料和等级资料采用例数、百分比进行描述,计数资料组间比较采用χ2检验,等级资料组间比较采用两独立样本秩和检验。P < 0.05为差异有统计学意义。
2. 结果
2.1 2组一般资料比较
2组PD患者在年龄、性别、PD病程、失眠病程以及Hoehn-Yahr分级等临床资料方面相比,差异均无统计学意义(P > 0.05),见 表1。
表 1 2组一般资料情况比较( $ \bar x \pm s$)Table 1. Comparison of clinical data between two groups ( $ \bar x \pm s$)项目 试验组(n = 33) 对照组(n = 33) χ2/t P 性别[n(%)] 0.061 0.805 男 15(45.5) 16(48.5) 女 18(54.5) 17(51.5) 年龄(岁) 63.06 ± 7.97 61.42 ± 9.72 −0.748 0.457 帕金森病病程(a) 4.33 ± 1.50 4.30 ± 1.56 −0.075 0.940 失眠病程[岁,M(P25,P75)] 2(1,6.5) 3(1,5.5) −0.155 0.877 Hoehn-Yahr分级[n(%)] −0.246 0.806 1级 5(15.2) 5(15.2) 1.5级 6(18.2) 6(18.2) 2级 13(39.4) 12(36.4) 2.5级 6(18.2) 5(15.2) 3级 3(9.1) 5(15.2) 2.2 2组患者干预前后PSQI、ISI评分比较
2组PD患者干预后的PSQI总分及ISI总分均较干预前有明显改善,组内差异均有统计学意义(P < 0.05),与对照组相比,试验组的PSQI总分及ISI总分明显降低,差异有统计学意义( P < 0.05),见 表2。
表 2 2组患者干预前后PSQI、ISI评分比较[( $ \bar x \pm s$),分]Table 2. Comparison of PSQI and ISI sores between two groups of Patients [( $ \bar x \pm s$),points]组别 n PSQI总分 ISI总分 干预前 干预后 干预前 干预后 试验组 33 12.21 ± 2.32 8.12 ± 1.93# 15.61 ± 3.22 9.88 ± 3.67# 对照组 33 12.12 ± 1.69 10.27 ± 1.84# 15.24 ± 3.54 13.40 ± 3.41# t 0.182 −4.630 0.437 −4.030 P 0.856 < 0.001* 0.664 < 0.001* 注:PSQI = 匹兹堡睡眠质量指数量表,ISI = 失眠严重指数量表。与同组干预前比较,#P < 0.05;组间比较, *P < 0.05。 2.3 2组患者干预前后睡眠日记各因子(SE、SOL、TST)比较
2组PD患者干预后的入睡潜伏期及总睡眠时间均较干预前有明显改善,组内差异均有统计学意义(P < 0.05),并且2组组间差异有统计学意义( P < 0.05)。试验组患者干预后睡眠效率较前有明显改善,组内差异有统计学意义( P < 0.05),2组组间差异无统计学意义( P > 0.05),见 表3。
表 3 2组患者干预前后睡眠日记各因子(SE、SOL、TST)比较( $ \bar x \pm s$)Table 3. Comparison of sleep diary factors (SE,SOL,TST) before and after intervention in 2 groups of patients ( $ \bar x \pm s$)组别 n SE(%) SOL(min) TST(min) 干预前 干预后 干预前 干预后 干预前 干预后 试验组 33 0.78 ± 0.10 0.87 ± 0.06# 44.61 ± 13.46 30.47 ± 7.13# 343.84 ± 78.48 407.17 ± 48.18# 对照组 33 0.76 ± 0.11 0.85 ± 0.06# 44.06 ± 19.24 36.36 ± 13.84# 324.95 ± 57.14 372.50 ± 35.20# t值 0.815 0.991 0.135 −2.164 1.413 3.338 P值 0.418 0.326 0.893 0.034* 0.163 0.001* 注:SE = 睡眠效率,SOL = 入睡潜伏期,TST=总睡眠时间。与同组干预前比较,#P < 0.05;组间比较, *P < 0.05。 2.4 2组患者干预前后HAMA14、HAMD17评分比较
2组PD患者干预后的HAMA14总分及HAMD17总分均较干预前有明显改善,组内差异均有统计学意义(P < 0.05)。与对照组相比,试验组的HAMA 14总分及HAMD17总分明显降低,差异有统计学意义(P < 0.05),见 表4。
表 4 2组患者干预前后HAMA14、HAMD17评分比较[( $ \bar x \pm s$),分]Table 4. Comparison of HAMA14 and HAMD17 sores between two groups of Patients [( $ \bar x \pm s$),points]组别 n HAMA14总分 HAMD17总分 干预前 干预后 干预前 干预后 试验组 33 13.03 ± 2.90 9.73 ± 2.21# 13.70 ± 5.37 10.00 ± 3.79# 对照组 33 13.30 ± 3.03 11.24 ± 2.50# 14.48 ± 4.14 12.39 ± 3.53# t −0.374 −2.607 −0.668 −2.656 P 0.710 0.011* 0.507 0.010* 注: HAMA14=汉密尔顿焦虑量表,HAMD17 = 汉密尔顿抑郁量表。与同组干预前比较,#P < 0.05;组间比较, *P < 0.05。 3. 讨论
3.1 CBT-I能够改善PD患者的睡眠质量
失眠是PD患者最常见的睡眠障碍[16],随着疾病逐渐加重,患者的失眠症状也随之严重。与普通失眠相比,PD患者睡眠障碍主要表现睡眠碎片化和浅表睡眠相对增加[17-18]。失眠的原因多种多样,可能与PD运动症状和非运动症状有关,包括夜间运动障碍、夜间肌张力障碍、僵硬、震颤、夜尿、抑郁、焦虑、痴呆以及多巴胺能药物的使用等,或者是存在原发性失眠[19-20]。失眠不仅会加重PD患者的疲劳程度,还会严重降低其生活质量[21]。
本研究结果显示,与对照组相比,干预后试验组PSQI量表及ISI量表得分明显降低,表明CBT-I能够提高PD患者的主观睡眠质量、降低失眠严重程度。这与Rios等[22]采用6周的CBT-I结合光疗对PD失眠严重程度指数的影响结果一致。在本研究中,CBT-I睡眠训练包括向患者讲授睡眠卫生教育、刺激控制疗法以及睡眠限制疗法相关知识,提升患者处理夜间失眠的正确方式,形成良好的睡眠卫生习惯,促进正性条件反射的形成,从而改善睡眠质量。CBT-I放松训练可以引导患者进行多层次多种类的放松,进而促进大脑睡眠-觉醒系统由觉醒向睡眠的转化。既往研究[23]已证实,放松训练和音乐疗法能有效控制慢性精神病患者的愤怒情绪,提高睡眠质量。进行渐进式肌肉放松训练可以有效缓解整体肌肉紧张,增进生理放松,缓解压力和焦虑,降低入睡潜伏期,改变睡眠结构,特别是增加慢波睡眠的总时间[24]。CBT-I行为训练能够强化睡眠认知的正确调整以促进认知与良好睡眠行为间的转化,打破负性情绪以及不良行为的恶性循环,最终促使患者形成稳定的睡眠行为习惯。在睡眠结构方面,本研究表明,8周的CBT-I能够改善患者的入睡潜伏期和总睡眠时间,而睡眠效率仅有改善趋势但没有统计学差异。而Yang等[25]采用4个月基于电话的CBT-I对22名PD失眠患者的研究发现,在治疗后和3个月的随访中,患者的总睡眠时间、睡眠潜伏期和睡眠效率均有改善。与本文结果不同,这可能与CBT-I干预时间较短、尚未完全起效有关。
3.2 CBT-I可有效改善PD患者的焦虑和抑郁情绪
PD患者抑郁的患病率为61%[26],焦虑的患病率从20%到40%不等[27]。PD患者焦虑和抑郁与失眠之间相互影响[28]。与无焦虑抑郁症状的PD患者相比,有焦虑抑郁症状的PD患者睡眠质量明显更差,睡眠障碍发生率更高。
本研究结果显示,CBT-I干预后,试验组患者焦虑、抑郁评分下降至(9.73±2.21)分、(10.00±3.79)分,表明CBT-I能有效降低PD患者的焦虑和抑郁水平。这与Lebrun等[29]应用单案例设计发现CBT-I对PD患者焦虑抑郁情绪的改善结果一致。由于失眠和焦虑抑郁障碍通常同时发生,治疗失眠可防止焦虑与抑郁症状共病的加重。据报道,CBT-I缓解患者负性情绪可能与增加前额皮质活动以及杏仁核、岛叶和前扣带皮层之间更好的耦合有关[30]。在本研究中,CBT-I通过让患者认识失眠发生的原因等相关知识,了解PD、失眠与焦虑抑郁之间相互影响的关系,促使其改变不良生活方式和心理状况,使其放松,从而降低其交感活性。CBT-I还通过进行多种方式的放松训练,影响脑的神经内分泌调节系统,使副交感神经活动占优势,达到缓解其负性情绪的目的。
目前,仅有少数调查研究了CBT-I对PD患者失眠的影响。我们的研究扩展了现有的关于CBT-I在PD患者临床实践中应用的文献,检验了其在PD失眠患者中的可行性和有效性。另外考虑到年龄问题,笔者的方案相对于计算机化的CBT-I而言,更适用于PD患者,并且可以为患者提供个性化的治疗方案。
综上所述,CBT-I可有效改善PD伴失眠患者的睡眠质量以及负性情绪。但本研究存在一定不足,本研究只采用睡眠日记及量表评测等方法进行结局测量,存在一定主观影响因素,后续研究可采用PSG等客观指标对患者睡眠情况进行监测。另外,本研究仅纳入1所三级甲等医院的住院患者,且Hoehn-Yahr分级为≤3级的患者,为轻中度PD患者,无法推广到全部的PD患者。建议未来进行覆盖各疾病阶段的多中心大样本研究来进一步验证本研究的结果。
-
表 1 LIFU对小鼠强迫游泳实验漂浮不动时间的影响(
$\bar x \pm s$ )Table 1. Effect of LIFU on floating immobility time of mice in forced swimming experiment (
$\bar x \pm s$ )组别 漂浮不动时间(s) t P 超声刺激组 46.29 ± 18.40 4.71 < 0.001 *** 对照组 16.67 ± 7.56 与对照组比较,***P < 0.001。 表 2 LIFU对小鼠旷场实验各项指标的影响(
$\bar x \pm s$ )Table 2. Effects of LIFU on various indicators of open field experiment in mice (
$\bar x \pm s$ )组别 行进总距离
(cm)行进平均速度(cm/s) 中央区活动距离(cm) 中央区停留时间(s) 中央区穿越次数(次) 超声刺激组 44.00 ± 17.81 0.15 ± 0.06 4.81 ± 3.06 16.35 ± 9.20 7.30 ± 4.08 对照组 67.79 ± 25.80 0.23 ± 0.09 11.11 ± 4.60 32.36 ± 11.15 17.10 ± 7.55 t −2.40 −2.40 −3.60 −3.50 −3.61 P < 0.05 * < 0.05 * < 0.01 ** < 0.01 ** < 0.01 ** 与对照组比较,*P < 0.05, **P < 0.01。 表 3 LIFU对小鼠水迷宫实验各项指标的影响(
$\bar x \pm s$ )Table 3. Effects of LIFU on various indicators of water maze experiment in mice (
$\bar x \pm s$ )组别 逃避潜伏时间(s) 穿越平台次数(次) 目标象限停留时间(s) 超声刺激组 32.50 ± 20.14 1.08 ± 0.72 22.40 ± 7.50 对照组 16.04 ± 4.55 2.38 ± 0.74 31.37 ± 6.63 t 2.52 −4.00 −2.83 P < 0.05 * < 0.001 *** < 0.05 * 与对照组比较,*P < 0.05, ***P < 0.001。 表 4 LIFU对小鼠单胺类神经递质含量的影响(
$\bar x \pm s$ )Table 4. Effects of LIFU on monoamine neurotransmitters in mice (
$\bar x \pm s$ )组别 DA(pg/mL) NE(ng/mL) 5-HT(ng/mL) 超声刺激组 25.69 ± 3.22 1.95 ± 0.19 46.83 ± 5.38** 对照组 33.52 ± 1.82 2.73 ± 0.25 78.19 ± 5.47 t −6.69 −7.41 −15.37 P < 0.0001 ** < 0.0001 ** < 0.0001 ** 与对照组比较,**P < 0.0001。 -
[1] Di Biase L,Falato E,Di Lazzaro V. Transcranial focused ultrasound (tFUS) and transcranial unfocused ultrasound (tUS) neuromodulation:from theoretical principles to stimulation practices[J]. Front Neurol,2019,10:549. doi: 10.3389/fneur.2019.00549 [2] Cui W,Aida T,Ito H. Dopaminergic signaling in the nucleus accumbens modulates stress-coping strategies during inescapable stress[J]. Neurosci,2020,40(38):7241-7254. doi: 10.1523/JNEUROSCI.0444-20.2020 [3] PuW,Jiaqi Z,Jiadan Y,et al. Brain modulatory effects by low-intensity transcranial ultrasound stimulation (TUS):a systematic review on both animal and human studies[J]. Front Neurosci,2019,13:696. doi: 10.3389/fnins.2019.00696 [4] Lee W,Lee S,Park M et al. Image-guided focused ultrasound-mediated regional brain stimulation in sheep[J]. Ultrasound in medicine & biology,2016,42(2):459-470. [5] Dallapiazza R,Timbie K,Holmberg S et al. Noninvasive neuromodulation and thalamic mapping with low-intensity focused ultrasound[J]. Journal of Neurosurgery,2018,128(3):875-884. doi: 10.3171/2016.11.JNS16976 [6] Davide F,Lennart V,Rogier B et al. Manipulation of subcortical and deep cortical activity in the primate brain using transcranial focused ultrasound stimulation[J]. Neuron,2019,101(6):1109-1116. doi: 10.1016/j.neuron.2019.01.019 [7] Lee W,Kim H C,Jung Y et al. Transcranial focused ultrasound stimulation of human primary visual cortex[J]. Sci Rep,2016,6(1):383-393. [8] Gibson B C,Sanguinetti J L,Badran B W et al. Increased excitability induced in the primary motor Cortex by transcranial ultrasound stimulation[J]. Front Neurol,2018,9:1007. doi: 10.3389/fneur.2018.01007 [9] Legon W,Bansal P,Tyshynsky R et al. Transcranial focused ultrasound neuromodulation of the human primary motor cortex[J]. Sci Rep,2018,8(1):10007. doi: 10.1038/s41598-018-28320-1 [10] Yoo S S,Bystritsky A,Lee J H et al. Focused ultrasound modulates region-specific brain activity[J]. Neuroimage,2011,56(3):1267-1275. doi: 10.1016/j.neuroimage.2011.02.058 [11] Kim H,Park M Y,Lee S D et al. Suppression of EEG visual-evoked potentials in rats through neuromodulatory focused ultrasound[J]. Neuroreport,2015,26(4):211-215. doi: 10.1097/WNR.0000000000000330 [12] 孟文. 低强度聚焦超声调控小鼠学习记忆的实验研究[D]. 北京: 中国科学院大学中国科学院深圳先进技术研究院硕士论文, 2020. [13] WeiTing L,RanChou C,WenWei L,et al. Protective effects of low-intensity pulsed ultrasound on aluminum-induced cerebral damage in Alzheimer’ s disease rat model[J]. Scientific Reports,2015,5(1):17-20. [14] Min B,Yang P,Bohlke M,et al. Focused ultrasound modulates the level of cortical neurotransmitters:potential as a new functional brain mapping technique[J]. Int J Imag Syst Tech,2011,21(2):232-240. doi: 10.1002/ima.20284 [15] Yang P,Kim H,Lee W,et al. Transcranial focused ultrasound to the thalamus is associated with reduced extracellular GABA levels in rats[J]. Neuropsychobiology,2012,65(3):153-160. doi: 10.1159/000336001 [16] Wang W W, Li L, Wu W, et al. Effects of ultrasound on behavior and dopamine content in striatum of parkinson’s disease model mouse[C]. Paris: Atlantis Press, 2017: 186-191. [17] Constans C,Ahnine H,Santin M. Non-invasive ultrasonic modulation of visual evoked response by GABA delivery through the blood brain barrier[J]. J Control Release,2020,318:223-231. doi: 10.1016/j.jconrel.2019.12.006 期刊类型引用(2)
1. 王喜月,肖梦鸽,王璇,王爱霞. 帕金森病患者症状网络分析及核心症状识别. 护士进修杂志. 2024(14): 1457-1462+1487 .  百度学术
百度学术2. 陈青怡,徐春梅. 诱导睡眠联合抗阻力运动改善老年帕金森病睡眠障碍的效果观察. 世界睡眠医学杂志. 2024(08): 1747-1749 .  百度学术
百度学术其他类型引用(1)
-






 下载:
下载:

 下载:
下载: