Voluntary Exercise Training Attenuated the Proliferation and Growth of Human Breast Cancer with BRCA1 Mutation by Regulating Caspase-3 Activity
-
摘要:
目的 探讨自主运动训练在乳腺癌发生发展中的作用及其机制。 方法 将携带原发性人乳腺癌基因1(breast cancer gene 1,BRCA-1)突变基因的癌细胞在体外进行培养,用连续14 d自主跑轮运动鼠血清以及静坐鼠血清处理BRCA-1突变肿瘤细胞,并将上述条件血清培养的乳腺癌细胞注射到BALB/c裸鼠皮下,观察经过不同处理后体内外BRCA-1突变乳腺癌细胞及移植瘤的情况。 结果 在体外,与静坐鼠血清处理组相比,自主运动鼠血清处理后抑制了BRCA-1突变乳腺癌细胞的活力、生长,促进其凋亡,差异具有统计学意义(P < 0.05);在体内,自主运动鼠血清培养的BRCA-1突变乳腺癌细胞移植瘤较静坐对照组体积减小、生长缓慢(P < 0.05),体内外自主运动组均呈现出增强的Caspase-3活性(P < 0.05)。 结论 自主跑轮运动可能通过增强Caspase3的活性、抑制了BRCA1突变乳腺癌细胞以及其在BALB/c裸鼠皮下异种移植瘤的增殖、促进肿瘤细胞凋亡,从而抑制其体内外的生长。 Abstract:Objective This study aims to explore the role and mechanism of voluntary exercise training in the development and progression of breast cancer. Methods Primary human Breast Cancer gene 1-mutation (BRCA-1 mutation) cancer cells were cultured in vitro, before treated with the serum of wheel running rats and sedentary rats for 14 consecutive days. The cells cultured in above conditions were injected subcutaneously into BALB/c nude mice to observe the in vitro and in vivo breast cancer cells with BRCA-1 mutation and tumor transplantation after different interventions. Results In vitro, compared with sedentary rat serum group, the activity and growth of BRCA-1 mutant breast cancer cells were inhibited and apoptosis was promoted after in the wheel running group. In vivo, the grafts of BRCA-1 mutated breast cancer cells cultured from in the wheel running group decreased in volume and grew slower compared with the sedentary group, and the wheel running group showed enhanced Caspase-3 activity both in vivo and in vitro. Conclusion Voluntary wheel running may attenuate the growth of BRCA1 mutant breast cancer cells and their proliferation and apoptosis in BALB/c nude mice by enhancing the activity of Caspase3, thus inhibiting the growth of breast cancer cells in vitro and in vivo. -
Key words:
- Breast cancer /
- Breast Cancer (BRCA) gene 1 /
- Voluntary running wheel /
- Proliferation /
- Apoptosis
-
随着生育政策及社会经济形态的转变,高龄产妇(年龄≥35岁)数量日益增多。在妊娠及分娩过程中易损伤到盆底结构及功能,患盆底功能障碍性疾病(pelvic fIoor dysfunction,PFD)风险较高。诸多研究发现[1-2],高龄产妇产后患盆底疾病风险远高于适龄产妇,且影响因素较多,一旦发生势必会对产妇生活质量造成严重影响,应给予高度重视。早期评估诊断盆底功能及结构变化对于预防及降低PFD疾病发生有着重要意义。盆底超声为评估PFD疾病的主要辅助检查技术之一,具有安全无创等优势。二维超声[3]、三维超声[4]为临床常用盆底超声技术,可量化评估观察盆底解剖结构形态学变化情况。但由于盆底结构较为复杂,误诊漏诊风险较高,仍需对超声技术诊断效能作进一步明确。基于此,本研究将对比盆底二维,三维超声评估适龄、高龄产妇产后早期盆底结构及功能的变化,旨在明确盆底超声诊断效能,为临床精准诊疗提供参考依据,现报道如下。
1. 资料与方法
1.1 一般资料
选择本院2021年7月至2022年12月就诊的产后早期(6~8周)高龄产妇86例作为观察组,选择同期就诊的产后早期适龄产妇50例作为对照组。观察组年龄35~43岁(38.67±4.35)岁;BMI(24.14±2.58)kg/m2;孕次0~3次(1.38±0.14)次,人工流产史6例;对照组年龄22~34岁(27.15±4.21)岁;BMI(23.97±2.64)kg/m2;孕次0~3次(1.29±0.18)次,人工流产史5例。两组除年龄资料以外各项资料进行匹配,差异无统计学意义(P > 0.05)。
纳入标准:(1)符合盆底超声诊断适应证[5];(2)高龄产妇年龄≥35岁;(3)单胎妊娠、足月、阴道自然分娩;(4)患者家属签署知情同意书。
排除标准:(1)妊娠前存在PFD疾病;(2)存在妊娠合并症、产后大出血、持续恶露、泌尿系统炎症者;(3)既往有盆腔手术史、占位性病变者;(4)产后接受盆底功能恢复治疗者。
1.2 方法
所有研究对象均接受盆底二维、三维超声诊断,所有操作均由同一位超声科医生完成。仪器选择:GE Voluson E10彩色多普勒超声诊断仪,并配备经阴道探头RM6C-D及编码对比成像软件。具体操作如下:叮嘱产妇排空大便,并保持膀胱适度充盈,选择截石体位,保持髋部微曲及轻度外展,充分暴露会阴部。检查者采用耦合剂均匀涂抹于探头表面上,并采用安全套包裹,将探头轻柔置入阴道,对其子宫双附件进行检查后,将其放置于受检者会阴部。首先进行二维超声扫描,选择盆底正中矢状切面(显示膀胱颈、膀胱、尿道、耻骨联合前下缘、后间隙等结构)进行扫描,观察盆底器官位置及运动情况,并于静息状态、Valsalva动作(深吸气后屏气向下用力,持续6 s)下测量膀胱颈位置(bladder neck position,BNP)、膀胱尿道后角(posterior urethravesical angel,PUA),并计算膀胱颈移动度(bladder neck descent,BND)、尿道旋转角(urethral rotation angel,URA)。接着开启三维超声扫描,进行盆底正中矢状切面、肛管横切面扫查(显示耻骨、直肠、尿道、阴道、肛门括约肌、肛提肌等结构),获取肛提肌裂孔图像,测量静息状态、Valsalva动作下肛提肌裂孔前后径 ( levator hiatal anteroposterior diameter,LHAP)、肛提肌裂孔左右径(levator hiatal lateral diameter,LHLP)、肛提肌裂孔面积(levator hiatal area,LHA)。
1.3 观察指标
(1)对比2组产妇盆底二维超声评估参数;(2)对比2组产妇盆底三维超声评估参数;(3)对比盆底二维、三维超声对适龄、高龄产妇产后PFD的诊断效能:以PFD疾病相关权威指南诊断标准(盆腔脏器脱垂[6]、压力性尿失禁等)为金标准,患PFD为阳性,未患为阴性,计算两种技术诊断灵敏度(真阳性/(真阳性+假阴性))、特异度(真阴性/(真阴性+假阳性))及准确性((真阳性+真阴性)/总样本数);(4)盆底二维、三维超声诊断图像。
1.4 统计学处理
将数据纳入SPSS23.0软件中分析,计量资料(盆底二维、三维超声评估参数)比较采用t检验,并以(
$\bar x \pm s$ )表示,计数资料(诊断效能)采用χ2检验,并以率(%)表示,(P < 0.05)为差异有统计学意义。2. 结果
2.1 2组产妇盆底二维超声评估参数比较
观察组静息状态BNP、PUA、Valsalva动作BNP水平、BND水平明显高于对照组(P < 0.05);而Valsalva动作PUA及URA水平对比差异无统计学意义(P > 0.05),见表1。
表 1 盆底二维、三维超声对适龄产妇产后PFD的诊断效能比较(n)Table 1. Comparison of the diagnostic efficacy of two-dimensional and three-dimensional ultrasound (n)诊断技术 金标准 灵敏度(%) 特异度(%) 准确性(%) 阳性 阴性 合计 二维超声 阳性 7 3 10 77.78(7/9) 92.68(38/41) 90.00(45/50) 阴性 2 38 40 合计 9 41 50 三维超声 阳性 8 1 9 88.89(8/9) 97.56(40/41) 96.00(48/50) 阴性 1 40 41 合计 9 41 50 χ2 − − − − 0.400 1.051 1.383 P − − − − 0.527 0.305 0.240 2.2 2组产妇盆底三维超声评估参数比较
观察组Valsalva动作LHAP、LHLP、LHA水平均明显高于对照组(P < 0.05);而静息状态LHAP、LHLP、LHA水平对比差异无统计学意义(P > 0.05),见表2。
表 2 2组产妇盆底二维超声评估参数比较($ \bar x \pm s $ )Table 2. Comparison of the two groups of puerpera pelvic floor two-dimensional ultrasound evaluation parameter between the two groups ($ \bar x \pm s $ )组别 n 静息状态 Valsalva动作 BND(cm) URA(°) BNP(cm) PUA(°) BNP(cm) PUA(°) 观察组 86 −2.34 ± 0.47* 120.47 ± 25.34* −0.59 ± 0.23* 137.45 ± 24.32 1.75 ± 0.35* 30.78 ± 6.53 对照组 50 −3.11 ± 0.56 104.68 ± 20.15 −1.74 ± 0.39 139.54 ± 25.47 1.37 ± 0.44 29.45 ± 5.84 t − 8.577 3.766 21.654 0.475 5.545 1.190 P − <0.001 <0.001 <0.001 0.636 <0.001 0.236 与对照组比较,*P < 0.05。 2.3 盆底二维、三维超声对高龄产妇产后PFD的诊断效能比较
50例适龄产妇中PFD患者9例,膀胱脱垂5例,压力性尿失禁4例,及阴道、直肠脱垂。盆底二维、三维对比无差异(P > 0.05)。86例高龄产妇中PFD患者27例,其中盆底脱垂15例,压力性尿失禁12例。盆底三维超声诊断灵敏度(92.59%)、准确性(91.53%)明显高于二维超声(70.37%、77.91%),P < 0.05,见表3、表4。
表 3 2组产妇盆底三维超声评估参数比较($ \bar x \pm s $ )Table 3. Comparison of pelvic floor three-dimensional ultrasound evaluation parameters between the two groups ($ \bar x \pm s $ )组别 n 静息状态 Valsalva动作 LHAP(cm) LHLP(cm) LHA(cm2) LHAP(cm) LHLP(cm) LHA(cm2) 观察组 86 5.45 ± 1.26 3.01 ± 0.84 16.41 ± 2.54 6.07 ± 1.34* 4.16 ± 1.22* 25.25 ± 2.76* 对照组 50 5.12 ± 1.23 3.07 ± 0.58 15.72 ± 2.36 5.54 ± 1.25 3.73 ± 0.85 20.66 ± 2.52 t − 1.486 0.447 1.567 2.279 2.200 9.649 P − 0.140 0.656 0.119 0.024 0.030 <0.001 与对照组比较,*P < 0.05。 表 4 盆底二维、三维超声对高龄产妇产后PFD的诊断效能比较(n)Table 4. Comparison of the diagnostic effcacy of two-dimensional and three-dimensional ultrasound of pelvic floor for PFD in older women (n)诊断技术 金标准 灵敏度(%) 特异度(%) 准确性(%) 阳性 阴性 合计 二维超声 阳性 19 11 30 70.37(19/27) 70.37(48/59) 77.91(67/86) 阴性 8 48 56 合计 27 59 86 三维超声 阳性 25 5 30 92.59(25/27) 91.53(54/59) 91.86(79/86) 阴性 2 54 56 合计 27 59 86 χ2 − − − − 4.418 2.603 6.525 P − − − − 0.036 0.107 0.011 2.4 盆底二维、三维超声诊断图像
适龄、高龄产妇患者盆底二维、三维超声诊断图像分别见图1、图2,其中A为静息状态下盆底正中矢状切面;B为Valsalva动作下盆底正中矢状切面;C为断层成像模式观察肛提肌连续性,层间距2.5 mm(中间3幅图依次显示耻骨联合开放、正在关闭、已关闭状态;D为肛提肌裂孔;E为断层成像模式观察肛门括约肌连续性,其中左侧为肛门内括约肌下缘,右侧为外括约肌上缘。
3. 讨论
妊娠及分娩为引起女性盆底结构及功能损伤的主要因素,妊娠期间由于子宫体积增长,机体为适应妊娠会出现支持盆腔器官组织过度延伸,达到一定程度时将肌肉将可能丧失收缩恢复能力;分娩过程中阴道周围支持组织受到牵拉、扩张,甚或肌肉纤维断裂,继而导致盆底肌损伤。而进行产后盆底功能检测对于PFD早期诊断及预防有着重要意义。盆底超声为临床产后检查评估盆底结构主要技术,可观察盆底解剖结构形态学变化,继而评估其组织功能状态,为临床诊治及疗效评估提供客观依据。二维经阴道超声是评估的主要筛查工具,具有简单、可重复性、成像清晰等特征,可用于评估膀胱、膀胱颈、尿道等组织形态变化,辅助盆底功能及结构评估[7-9]。三维超声具有较高空间分辨率,可通过多平面成像及图像重建后处理,为临床评估盆底结构及功能提供可靠数据[10-12]。
本研究显示,观察组静息状态BNP、PUA、Valsalva动作BNP水平、BND水平明显高于对照组(P < 0.05);而Valsalva动作PUA及URA水平对比差异无统计学意义(P > 0.05)。观察组Valsalva动作LHAP、LHLP、LHA水平均明显高于对照组(P < 0.05);而静息状态LHAP、LHLP、LHA水平对比差异无统计学意义(P > 0.05)。说明相较于适龄产妇,高龄产妇产后早期更易出现肛提肌、膀胱等盆底组织功能及结构变化,适龄产妇变化不大。李宁等[13]研究报道,高龄产妇的Valsalva动作LHA水平高于适龄产妇,该结果与本研究结果一致。其原因在于相较于适龄产妇,高龄产妇的生理功能出现逐步下降,尤其是盆底肌群收缩反应时间延长,速度减慢,盆底肌肉、神经长时间处于压迫状态,出现盆底组织损伤概率较高;此外盆底肌肉组织中胶原、弹性蛋白含量降低,无法维持正常收缩功能,出现超声异常征象。盆底三维超声诊断灵敏度、准确性明显高于二维超声。此处已删减研究报道,孕妇的盆底肌肉不仅受到与分娩相关的机械损伤的影响,还受到怀孕期间生理变化的影响,继而使得提肌裂孔增大[14]。其原因在于虽然二维超声可以全面评估显示盆底结构,但该技术无法显示示肛提肌、盆膈裂孔等结构,而三维超声可通过多平面成像及图像重建后处理,更加准确、立体显示盆底结构组织及结构变化,该技术对于测量肛提肌裂孔各参数有着较高精确度,其中肛提肌裂孔可进一步反映肛提肌顺应性,继而提高临床诊断效能[15]。
综上所述,高龄产妇产后早期更易出现盆底功能及结构变化,相较于二维超声,盆底三维超声更有助于提高PFD诊断效能。
-
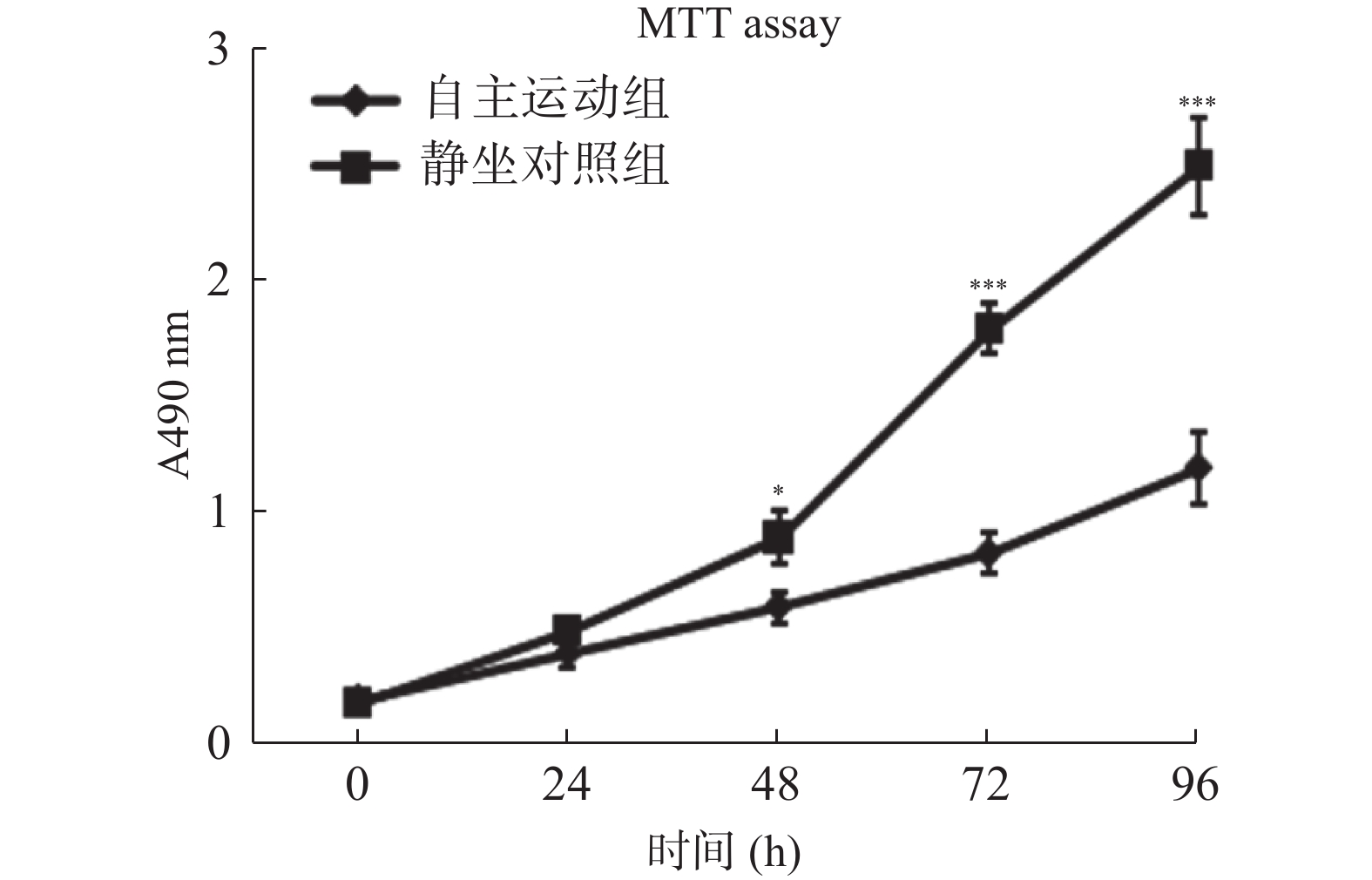
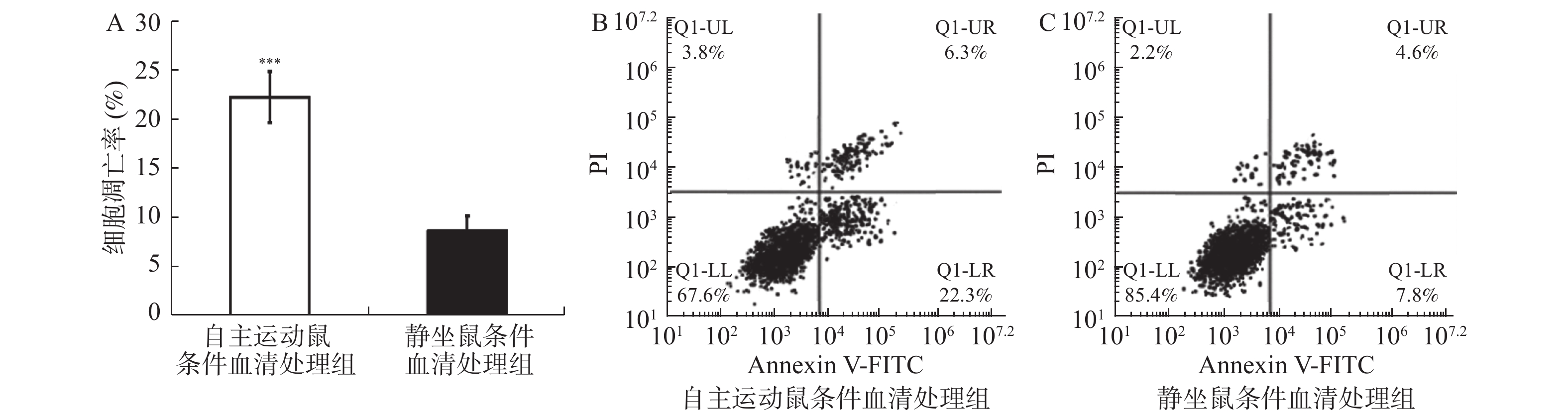
图 3 自主跑轮运动鼠条件血清处理促进了乳腺癌细胞的凋亡
流式细胞术检测自主运动鼠条件血清及静坐鼠条件血清处理后BRCA1-mut细胞在体外的凋亡情况。A:2组乳腺癌BRCA1-mut细胞的凋亡率比较;B:2组经流式检测显示乳腺癌BRCA1-mut细胞呈现不同的对PI(提示细胞死亡)与Annexin V-FITC荧光染料(提示细胞凋亡)的着色比率,自主运动鼠条件血清处理后有更多的细胞发生凋亡。与静坐鼠条件血清处理组相比:*P < 0.05,**P < 0.01,***P < 0.001。
Figure 3. Conditioned serum treatment promotes apoptosis of breast cancer cells
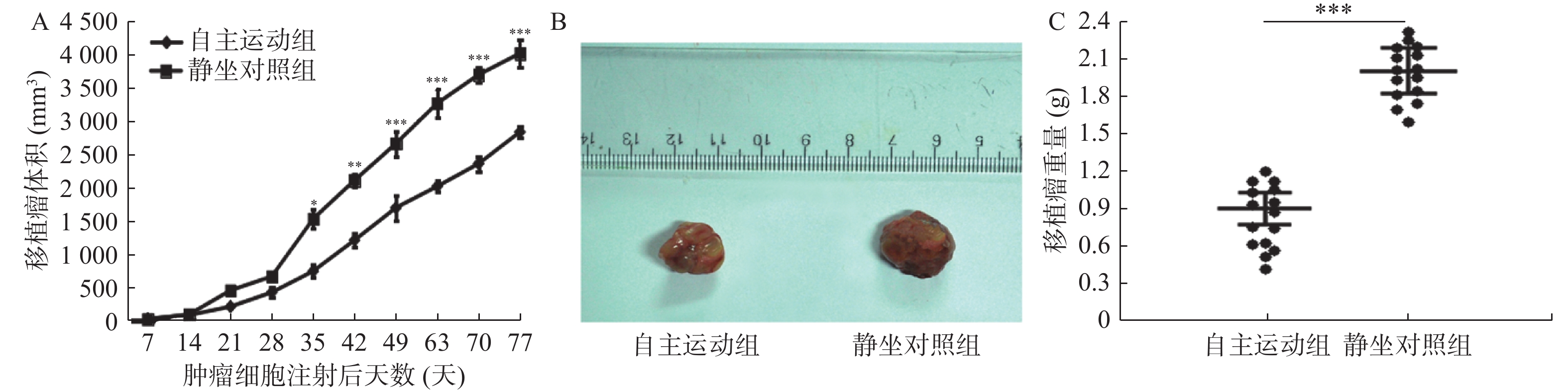
图 4 自主跑轮运动抑制了BRCA1-mut乳腺癌移植瘤在裸鼠体内的生长
A:自BRCA1-mut细胞皮下注射开始至第77天期间2组裸鼠移植瘤的体积比较;B:BRCA1-mut细胞皮下移植注射后77天分离2组裸鼠皮下肿瘤的代表性对比图;C:BRCA1-mut细胞皮下注射后第77天观察结束时2组裸鼠移植瘤的重量比较。与静坐对照组相比:*P < 0.05,**P < 0.01,***P < 0.001。
Figure 4. Voluntary running wheel exercise inhibites the growth of BRCA1-mut breast cancer grafts in nude mice
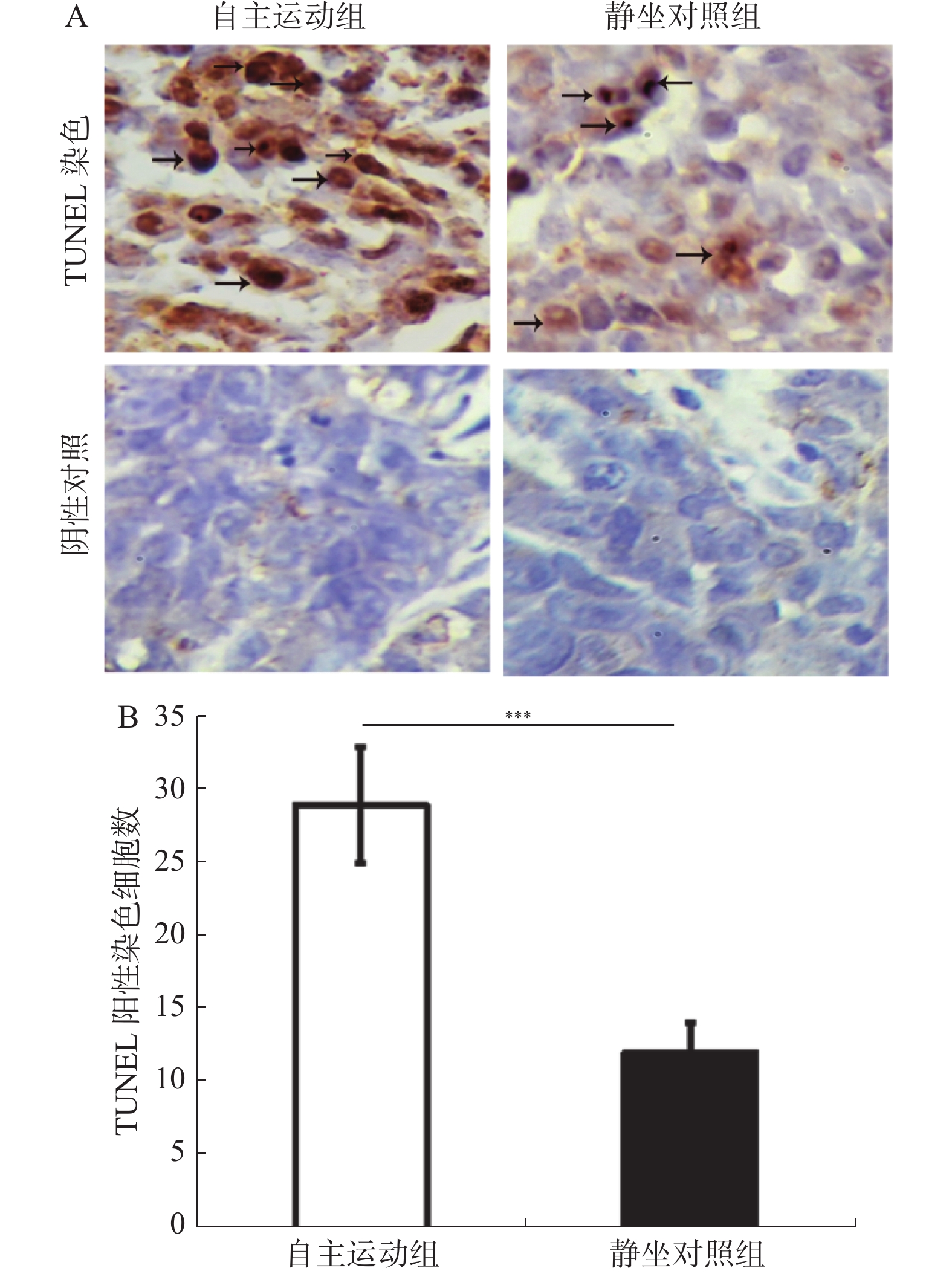
图 5 自主跑轮运动诱导了BRCA1-mut乳腺癌移植瘤的凋亡(×400)
自主运动对BRCA1-mut乳腺癌细胞裸鼠移植瘤细胞凋亡的检测,用TUNEL末端标记法对各组凋亡情况做检测。A:各组组织TUNEL染色着色细胞,箭头标示的为TUNEL阳性染色细胞,放大倍数为400倍;B:各组TUNEL阳性染色细胞计数结果。与静坐对照组相比:*P < 0.05,**P < 0.01,***P < 0.001。
Figure 5. Voluntary running wheel induces the apoptosis of the BRCA1-mut breast cancer cells transplanted tumors in the nude mice (×400)
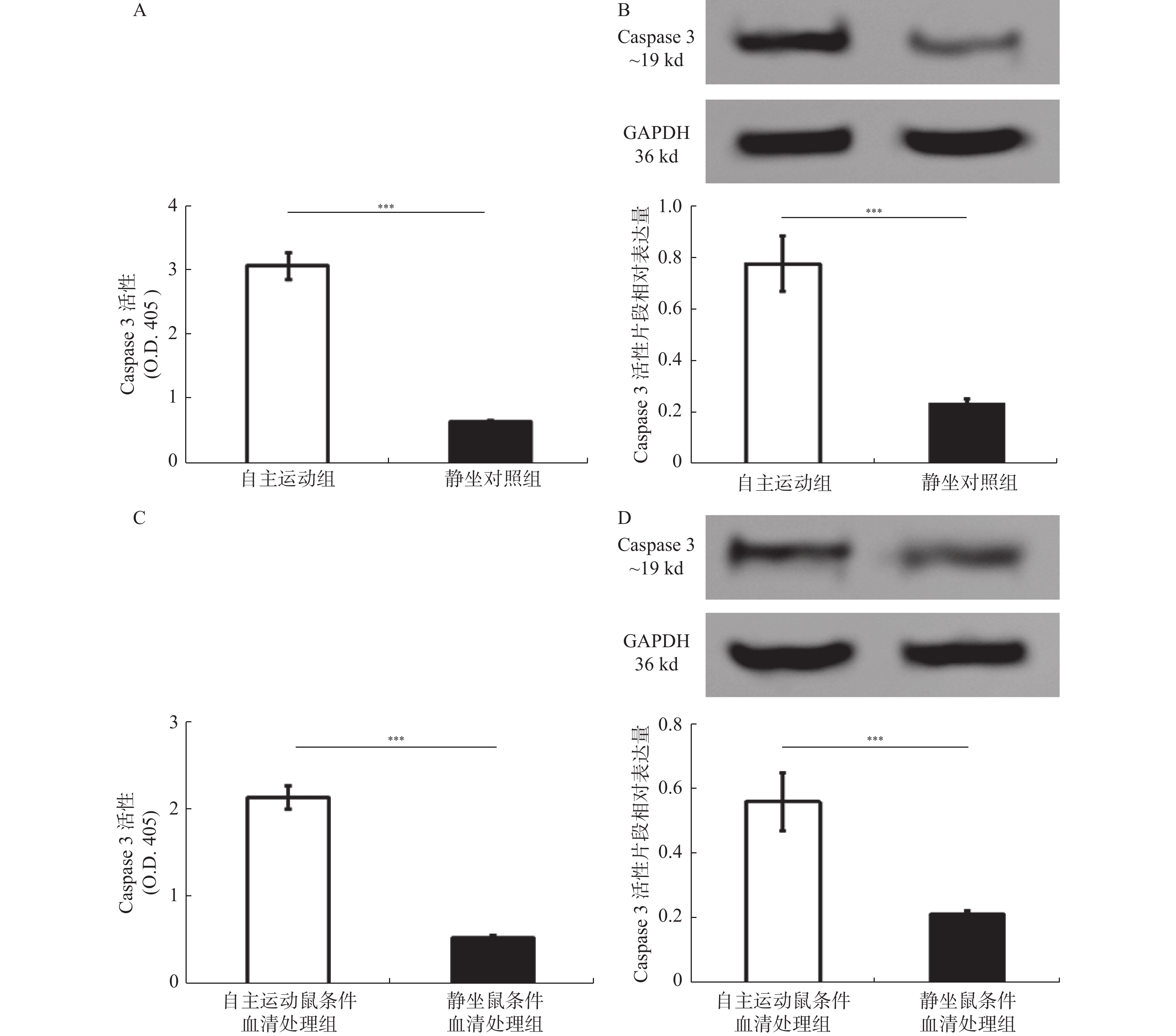
图 6 自由运动激活BRCA1-mut乳腺癌细胞及移植瘤的Caspase 3
自主运动对体外培养的BRCA1-mut乳腺癌细胞及裸鼠移植瘤内Caspase 3活性及活化片段的影响。A:自主运动组与静坐对照组中移植肿瘤内Caspase 3的活性比较;B:自主运动组与静坐对照组中移植肿瘤内Caspase 3活性片段的表达检测;C:自主运动鼠条件血清及静坐鼠条件血清处理后BRCA1-mut细胞Caspase 3的活性比较;D:自主运动鼠条件血清及静坐鼠条件血清处理后BRCA1-mut细胞Caspase 3活性片段的表达检测。O.D.405,405 nm 波长下酶标仪检测到的光密度值。与静坐鼠条件血清处理组相比:*P < 0.05,**P < 0.01,***P < 0.001。
Figure 6. Voluntary running wheel increases the Caspase 3 activity in either of BRCA1-mut breast cancer cells and transplanted tumorsin nude mice
表 1 携带BRCA1突变基因的乳腺癌患者BRCA1的突变状况及信息
Table 1. BRCA1 gene mutation status and clinical information of breast cancer patients
BRCA1 突变类型(n) 家庭表型(n) 患者年龄(岁)(n) 组织学分类(n) 肿瘤分期(0-Ⅳ期)(n) K679X(5) family BC#(16) < 30(1) 小叶型(5) ⅡA&(5) 185delAG(4) family OC(2) 30~35(8) 导管型(25) ⅡB(5) E1353X(5) family BC及 OC(1) 36~40(2) 混合型(1) ⅢA(8) 5382insC(4) 无(12) 41~45(6) ⅢB(7) S770X(4) 46~50(7) ⅢC(5) S868X(3) 51~55(4) Ⅳ(1) 231delAA(3) 56~60(2) 8548delAAGG(1) >60(1) 335delA(1) IVS17+2T>C(1) #,家庭表型,包括:家族性乳腺癌(family breast cancer,family BC),家族性卵巢癌(family ovarian cancer,family OC);&,乳腺癌分期根据国际通用的UICC (union for international cancer control) 以及AJCC (American joint committee on cancer) 标准进行[11]。 -
[1] Schünemann H J,Lerda D,Quinn C,et al. Breast cancer screening and diagnosis: A synopsis of the European breast guidelines[J]. Ann Intern Med,2020,172(1):46-56. doi: 10.7326/M19-2125 [2] Kharel S, Shrestha S, Yadav S, et al. BRCA1/BRCA2 mutation spectrum analysis in South Asia: A systematic review[J]. J Int Med Res, 2022, 50(1): 3000605211070757. [3] Friebel T M,Domchek S M,Rebbeck T R. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: Systematic review and meta-analysis[J]. Journal of the National Cancer Institute,2014,106(6):dju091. [4] Dubeau L. Pathogenesis of serous,extra-uterine Müllerian epithelial cancer and therapeutic implications[J]. Transl Cancer Res,2015,4(1):3-13. [5] Kamińska M,Ciszewski T,Łopacka-Szatan K,et al. Breast cancer risk factors[J]. Przegla̜d Menopauzalny,2015,14(3):196-202. [6] Waxman A,World Health Assembly. WHO global strategy on diet,physical activity and health[J]. Food Nutr Bull,2004,25(3):292-302. doi: 10.1177/156482650402500310 [7] García-Chico C,López-Ortiz S,Peñín-Grandes S,et al. Physical exercise and the hallmarks of breast cancer: A narrative review[J]. Cancers (Basel),2023,15(1):324. doi: 10.3390/cancers15010324 [8] Christensen J F,Jones L W,Andersen J L,et al. Muscle dysfunction in cancer patients[J]. Annals of Oncology,2014,25(5):947-958. doi: 10.1093/annonc/mdt551 [9] Ju J,Nolan B,Cheh M,et al. Voluntary exercise inhibits intestinal tumorigenesis in Apc(Min/+) mice and azoxymethane/dextran sulfate sodium-treated mice[J]. BMC Cancer,2008,2(8):316. [10] Lu Y P,Lou Y R,Nolan B,et al. Stimulatory effect of voluntary exercise or fat removal (partial lipectomy) on apoptosis in the skin of UVB light-irradiated mice[J]. Proc Natl Acad Sci U S A,2006,103(44):16301-16306. doi: 10.1073/pnas.0607789103 [11] Kostourou V,Cartwright J E,Johnstone A P,et al. The role of tumour-derived iNOS in tumour progression and angiogenesis[J]. Br J Cancer,2011,104(1):83-90. doi: 10.1038/sj.bjc.6606034 [12] National research council (US) institute for laboratory animal research. Guide for the care and use of laboratory animals[M]. Washington (DC): National Academies Press (US), 1996: 37-88. [13] Hojman P,Dethlefsen C,Brandt C,et al. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth[J]. Am J Physiol Endocrinol Metab,2011,301(3):504-510. doi: 10.1152/ajpendo.00520.2010 [14] Hu T,Zhou F J,Chang Y F,et al. miR21 is associated with the cognitive improvement following voluntary running wheel exercise in TBI mice[J]. J Mol Neurosci,2015,57(1):114-122. doi: 10.1007/s12031-015-0584-8 [15] Minemura H,Takagi K,Miki Y,et al. Abnormal expression of miR-1 in breast carcinoma as a potent prognostic factor[J]. Cancer Sci,2015,106(11):1642-1650. doi: 10.1111/cas.12808 [16] Hu T,Zhang C,Tang Q,et al. Variant G6PD levels promote tumor cell proliferation or apoptosis via the STAT3/5 pathway in the human melanoma xenograft mouse model[J]. BMC Cancer,2013,13:251. doi: 10.1186/1471-2407-13-251 [17] Gordon C J,Phillips P M,Johnstone A F. Impact of genetic strain on body fat loss,food consumption,metabolism,ventilation,and motor activity in free running female rats[J]. Physiol Behav,2016,153(1):56-63. [18] Otsuka A,Shiuchi T,Chikahisa S,et al. Voluntary exercise and increased food intake after mild chronic stress improve social avoidance behavior in mice[J]. Physiol Behav,2015,151(1):264-271. [19] Pagliusi M Jr,Bonet I J M,Brandão A F,et al. Therapeutic and preventive effect of voluntary running wheel exercise on social defeat stress (SDS)-induced depressive-like behavior and chronic pain in mice[J]. Neuroscience,2020,428(4):165-177. [20] Sartori C R,Pagliusi M Jr,Bonet I J M,et al. Running wheel exercise induces therapeutic and preventive effects on inflammatory stimulus-induced persistent hyperalgesia in mice[J]. PLoS One,2020,15(10):e0240115. doi: 10.1371/journal.pone.0240115 [21] Reddy B S, Sugie S, Lowenfels A. Effect of voluntary exercise on azoxymethane-induced colon carcinogenesis in male F344 rats[J]. Cancer Research, 1998, 48(24 Part 1): 7079-7081. [22] Lu M,Sanderson S M,Zessin A,et al. Exercise inhibits tumor growth and central carbon metabolism in patient-derived xenograft models of colorectal cancer[J]. Cancer Metab,2018,6(3):14. [23] Cohen L A,Kendall M E,Zang E,et al. Modulation of N-nitrosomethylurea-induced mammary tumor promotion by dietary fiber and fat[J]. J Natl Cancer Inst,1991,83(7):496-501. doi: 10.1093/jnci/83.7.496 [24] Zhou Y,Jia N,Ding M,et al. Effects of exercise on inflammatory factors and IGF system in breast cancer survivors: A meta-analysis[J]. BMC Womens Health,2022,22(1):507. doi: 10.1186/s12905-022-02058-5 [25] Agostini D,Natalucci V,Baldelli G,et al. New insights into the role of exercise in inhibiting mTOR signaling in triple-Negative breast cancer[J]. Oxid Med Cell Longev,2018,2018:5896786. [26] Theriau C F,Connor M K. Voluntary physical activity counteracts the proliferative tumor growth microenvironment created by adipose tissue via high-fat diet feeding in female rats[J]. Physiol Rep,2017,5(13):e13325. doi: 10.14814/phy2.13325 [27] Pedersen L,Idorn M,Olofsson G H,et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution[J]. Cell Metab,2016,23(3):554-562. doi: 10.1016/j.cmet.2016.01.011 [28] Thompson H J. Effect of exercise intensity and duration on the induction of mammary carcinogenesis[J]. Cancer Res, 1994, 54(7 Suppl): 1960s-1963s. [29] Steiner J L,Davis J M,McClellan J L,et al. Effects of voluntary exercise on tumorigenesis in the C3(1)/SV40Tag transgenic mouse model of breast cancer[J]. Int J Oncol,2013,42(4):1466-1472. doi: 10.3892/ijo.2013.1827 [30] Welsch M A,Cohen L A,Welsch C W. Inhibition of growth of human breast carcinoma xenografts by energy expenditure via voluntary exercise in athymic mice fed a high-fat diet[J]. Nutrition and Cancer,1995,23(3):309-318. doi: 10.1080/01635589509514385 期刊类型引用(6)
1. 郑秀艳,陈曦,何扬波,黄磊. 干燥方式对红托竹荪品质特性和微观结构的影响. 现代食品科技. 2024(03): 209-218 .  百度学术
百度学术2. 任梽源,刘新硕,陆时金,杨文智,李海鹰. 谷氨酸铁螯合物对缺铁性贫血小鼠的补铁效果评价. 河北大学学报(自然科学版). 2024(04): 365-372 .  百度学术
百度学术3. 李琳,陈丹,范晓禹,郑喜群,崔素萍. 英国红芸豆抗氧化肽营养价值评价及体外模拟消化分析. 黑龙江八一农垦大学学报. 2024(04): 62-70 .  百度学术
百度学术4. 杨皓博,周铭晗,范文涛. 谷氨酸转运体GLT调控星形胶质细胞与神经元交互作用对癫痫发作的影响. 中国实用神经疾病杂志. 2023(02): 239-244 .  百度学术
百度学术5. 谭智霖,谢琦,廖炎辉,王雅杰,杜磊,陈惠娴. 轻度认知障碍额叶白质~1H-MRS与认知功能的相关性. 磁共振成像. 2021(08): 6-10 .  百度学术
百度学术6. 王慧,段旭艳,陈鲁曼. 产前应激对子代抑郁的影响及其机制的研究进展. 菏泽医学专科学校学报. 2021(03): 85-88 .  百度学术
百度学术其他类型引用(5)
-






 下载:
下载:
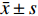
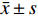
 下载:
下载: