Effect of MiR-26a-5p Regulating SLC26A4 to Ameliorate Hearing Loss in Deafness
-
摘要:
目的 检测miR-26a-5p和SLC26A4在耳聋小鼠和听力损失小鼠中的表达变化,以此研究miR-26a-5p 调控SLC26A4挽救听力减退的作用。 方法 通过构建小鼠听力损失模型和小鼠耳聋模型,使用听性脑干反应测试检测小鼠右耳的听力来鉴定模型的构建是否成功。使用qPCR实验检测miR-26a-5p和SLC26A4在小鼠听力损失和耳聋后的相对表达量,通过TargetScan网站预测miR-26a-5p靶向结合SLC26A4的具体位点,对小鼠注射miR-26a-5p和SLC26A4的干扰和过表达载体,继续检测miR-26a-5p和SLC26A4的表达变化。 结果 在小鼠听力损失和耳聋后,miR-26a-5p表达显著降低(P < 0.05),而SLC26A4表达明显升高(P < 0.05),在小鼠注射antagomir后,miR-26a-5p明显下调(P = 0.047),同时SLC26A4显著上调(P = 0.014),同时小鼠听力阈值明显降低(P < 0.05),而在注射miR-26a-5p agomir后结果恰恰相反。 结论 miR-26a-5p可以通过SLC26A4从而降低听力减退以此抑制耳聋的发生。 -
关键词:
- 听力减退 /
- 耳聋 /
- miR-26a-5p /
- SLC26A4
Abstract:Objective To explore the effect of miR-26a-5p in regulating SLC26A4 to amiliorate hearing loss, and to detect the expression changes of miR-26a-5p and SLC26A4 in deaf mice and hearing loss mice. Methods A mouse hearing loss model and a mouse deafness model were constructed, and the auditory brainstem response test was used to detect the hearing in the right ear of mice to determine whether the models were successfully constructed. qPCR test was used to detect the relative expression of miR-26a-5p and SLC26A4 after hearing loss and deafness in mice were established. The specific site of miR-26a-5p targeting binding to SLC26A4 was predicted at TargetScan website. The interference and overexpression vector of miR-26a-5p and SLC26A4 were injected, and the expression changes of miR-26a-5p and SLC26A4 were detected. Results After hearing loss and deafness were established, the expression of miR-26a-5p was significantly decreased (P < 0.05), while the expression of SLC26A4 was significantly increased (P < 0.05). After antagomir injection, miR-26a-5p was significantly down-regulated (P = 0.047), while SLC26A4 was significantly up-regulated (P = 0.014); and the hearing threshold of mice decreased significantly (P < 0.05), but the result was just the opposite after injection of miR-26a-5p agomir. Conclusion miR-26a-5p can ameliorate hearing loss through SLC26A4 and thus decelerate the development of deafness. -
Key words:
- Hearing loss /
- Deafness /
- miR-26a-5p /
- SLC26A4
-
听力损失影响着全球约15亿人,而在中国所有年龄段中就有约2亿多人,这些病例中有大约一半被认为是遗传性的[1-2]。在遗传性耳聋的基因变异中,SLC26A4是除GJB2外最常见的耳聋致病基因,是导致大前庭导水管综合征(large vestibular aquduct syndrome,LVAS)的致病基因,在聋人中占19.4%,正常人群中的携带率为2%,典型表现为儿童时期的听力损失,90%的患者为双侧性,听力损失程度不一,病程一般为进行性或波动性听力下降,在跌倒、撞击等行为时易致明显的不可逆的听力下降[3]。在此种情况下,找到能对SLC26A4基因进行调控的方法就显得尤为重要。目前对于听力损失的治疗方案仍然局限于声音放大和人工耳蜗植入,但是听力恢复远非完美,特别是对嘈杂环境中的声音的感知[4-5]。这就迫切需要找到针对人类听力损失的生物治疗方法,去寻找并开发针对于各种类型听力损失的基因、细胞和药物疗法,从而为广大听力损失患者提供新的治疗手段和决策。miRNAs已被证实广泛存在于不同的细胞和组织中,在细胞周期、细胞凋亡、肿瘤发生和神经发生等生物学过程中发挥重要作用,有许多研究表明miRNA的异常导致了耳聋的发生,而关于miR-26a-5p与耳聋的机制研究非常少,因此,本文拟对miR-26a-5p 调控SLC26A4表达在听力减退中的作用做一研究,以期能为SLC26A4基因突变导致的耳聋的治疗提供一定参考依据。
1. 材料与方法
1.1 实验材料
1.1.1 动物及分组
本实验所使用的SPF级别雄性C57小鼠(标准体重)均购自于昆明医科大学实验动物学部,本研究的实验方法和操作方法均已得到昆明医科大学实验动物福利委员会的批准。所有小鼠都饲养于实验动物学部,饲养环境均符合相关标准。18只C57小鼠被分为3组,为假手术组、听力损伤组和耳聋组。假手术组是注射生理盐水,而不注射致聋药物D-半乳糖。
1.1.2 实验试剂
D-半乳糖购自于山东萍聚生物科技有限公司,氯化钠注射液(Nacl)购自于云南南诏药业有限公司,无水乙醇、三氯甲烷、异丙醇均购自于天津市瑞明威化工有限公司,反转录试剂盒(PerfectStartUniRTKIT)和qPCR扩增试剂盒(PerfectStartUnigPCRKIT)均购自于昆明云科生物技术有限公司,异氟烷购自于深圳瑞沃德生命科技有限公司,miR-26a-5p和SLC26A4引物均购自于安徽九德科技有限公司,miR-26a-5p和SLC26A4的干扰和过表达载体均购自于广州锐博生物有限公司。
1.1.3 实验设备
高速低温离心机购自于湖南赫西仪器装备有限公司,荧光定量PCR仪和酶标仪购自于美国伯乐公司,TDT听觉诱发电位工作站购自于北京爱生科贸有限公司。
1.2 实验方法
1.2.1 模型构建
参照郝帅的D-半乳糖造模方法[6]进行一定改良,观测指标为:小鼠活动是否迟缓,小鼠双侧耳廓反应是否灵敏。对听力损伤模型制造组给予100 mg/kg的量进行腹腔注射给D-半乳糖,耳聋模型制造组给D-半乳糖150 mg/kg的量,假手术组根据小鼠的体重比例,腹腔注射同等量的Nacl注射液。以上注射时间均为每天2次,持续1个月。
1.2.2 听性脑干反应测试(ABR)
本实验在隔声屏蔽室内进行测试,通过不同频率的刺激信号在8 kHz、16 kHz、24 kHz和32 kHz时诱导小鼠在麻醉状态下的电生理反应,仪器通过平均技术进行相关信号处理,通过解析听性脑干反应测试频谱中8个谐波的相位和幅度,采用序贯检验的方法来消除持续的EEG噪声和诊断听力的其他干扰。刺激声音强度以5 dB依次递减,如果能分辨出最低刺激强度的即为ABR阈值,重复3次。
1.2.3 注射治疗
对听力损伤和耳聋模型小鼠进行尾静脉注射miR-26a-5p和SLC26A4的干扰和过表达载体,每天1次,持续10 d。
1.2.4 小鼠耳蜗取材
在实验达到终止阶段,对所有小鼠进行异氟烷吸入过度麻醉处死,然后取出小鼠两侧听泡,放入液氮中保存用于后续qPCR实验。
1.2.5 qPCR实验
在Pubmed官网上下载miR-26a-5p及内参U6和SLC26A4及内参β-actin的核酸序列,通过PrimerPremier 6软件对它们的引物序列进行设计,将引物序列发九德科技有限公司进行合成,具体序列见表1。使用电动研磨液对小鼠的耳蜗进行研磨处理,使用Trizol法对耳蜗组织进行总RNA的提取。得到总RNA后,使用酶标仪测定RNA的浓度及纯度,并根据反转录试剂盒PerfectStartUniRTKIT说明书进行逆转录反应,再根据荧光定量试剂盒PerfectStartUnigPCRKIT说明书进行PCR扩增反应,得到PCR扩增的CT值后根据2-△△CT公式来计算miR-26a-5p和SLC26A4的相对表达量。
表 1 引物序列Table 1. Primer sequence基因名称 上游引物序列(5′- 3′) 下游引物序列(5′- 3′) β-actin GTGGGGCGCCCCAGGCACCA CTCCTTAATGTCACGCACGATTT SLC26A4 GCATCCTCTCCATTATCTACA TCCTTAACAGCCATACAGAC miR-26a-5p 通用引物 AGCCAGCGTTCAAGTAATCCAG U6 通用引物 ATGGACTATCATATGCTTACCGTA 1.3 统计学处理
所有的实验数据都使用SPSS 21.0软件进行统计分析,通过独立样本t检验统计2组之间(antagomir和agomir注射后小鼠miR-26a-5p和SLC26A4的相对表达量)的数据,通过单因素方差分析统计3组及3组以上(18只小鼠听性脑干测试及小鼠miR-26a-5p和SLC26A4的相对表达量)的数据,而后根据方差齐性检验比较组内差异,P < 0.05即表示差异具有统计学意义。
2. 结果
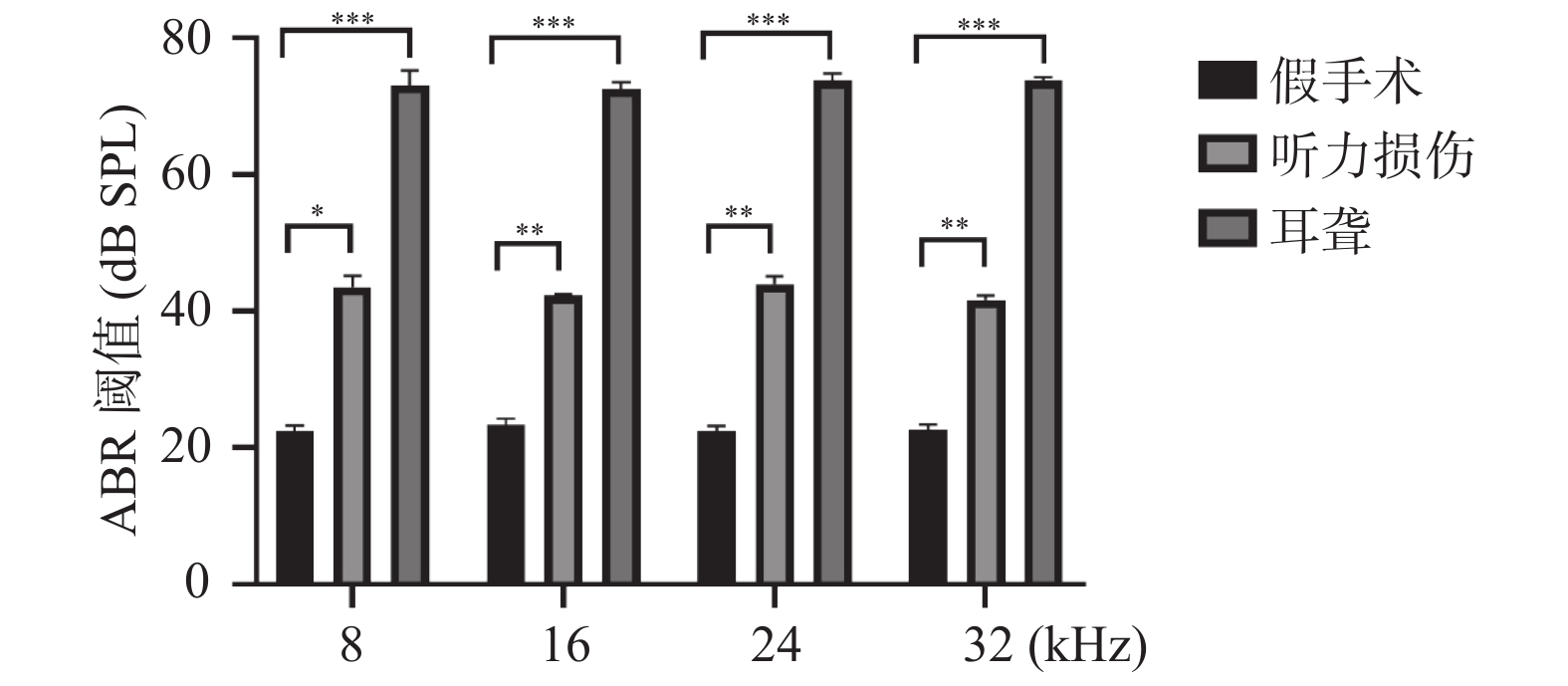
2.1 小鼠听力损伤模型和小鼠耳聋模型构建成功
使用听性脑干反应测试来检测各组C57小鼠的右耳听力情况,结果显示,无论是8 kHz、16 kHz、24 kHz还是32 kHz,同组之间的小鼠之间的听力阈值无明显差异,但是相对于假手术组来说,听力损伤和耳聋小鼠听力阈值明显提高,耳聋小鼠阈值有显著的翻倍,见图1,结果表明模型构建成功。
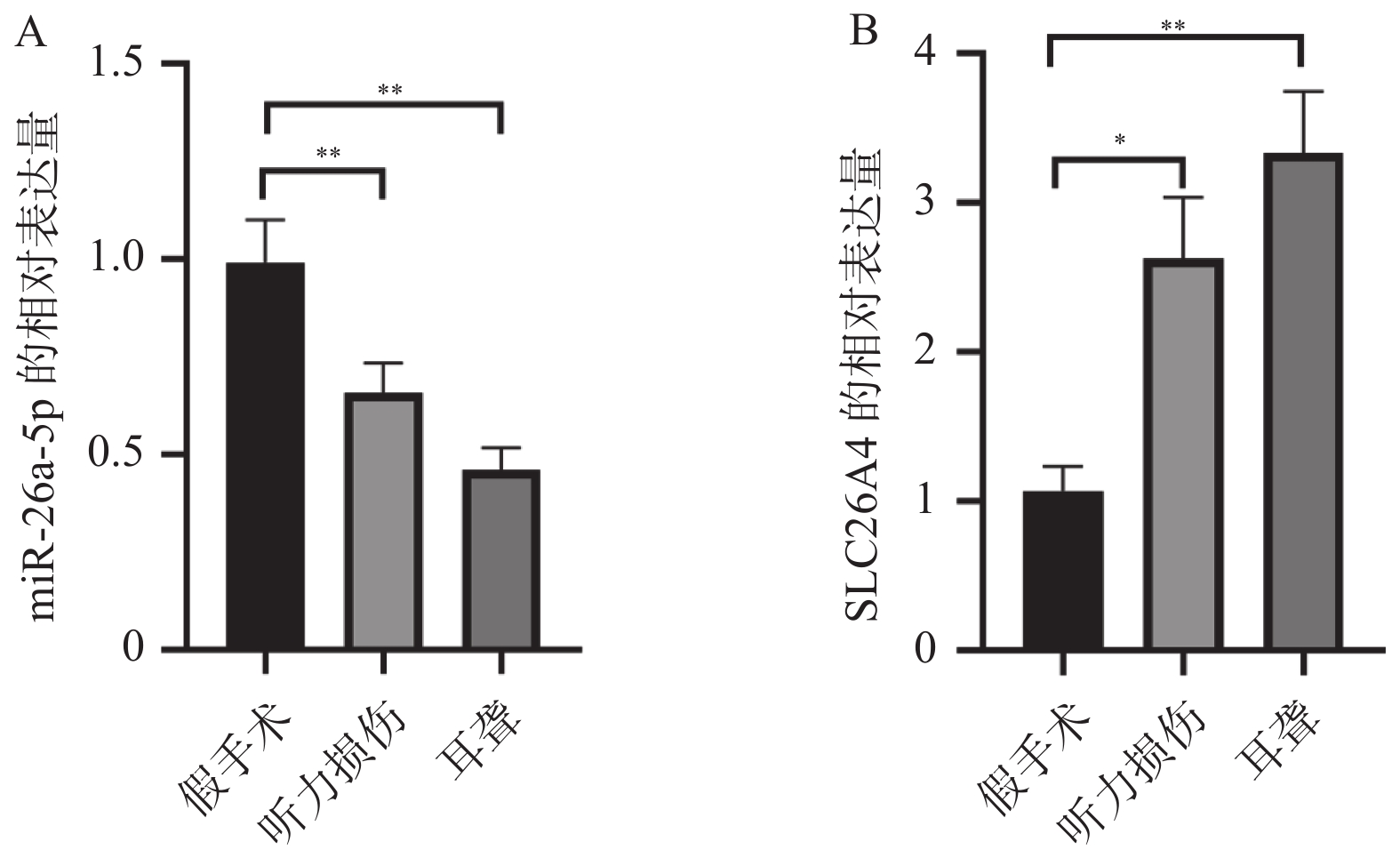
2.2 小鼠听力损伤和耳聋后miR-26a-5p下调和SLC26A4上调
在使用qPCR实验检测小鼠听力损伤和耳聋后耳蜗中miR-26a-5p和SLC26A4的表达情况后发现,与假手术组相比,miR-26a-5p在小鼠听力损伤和耳聋后表达异常,出现明显降低,见图2A;相反的是SLC26A4的表达却明显升高,见图2B。
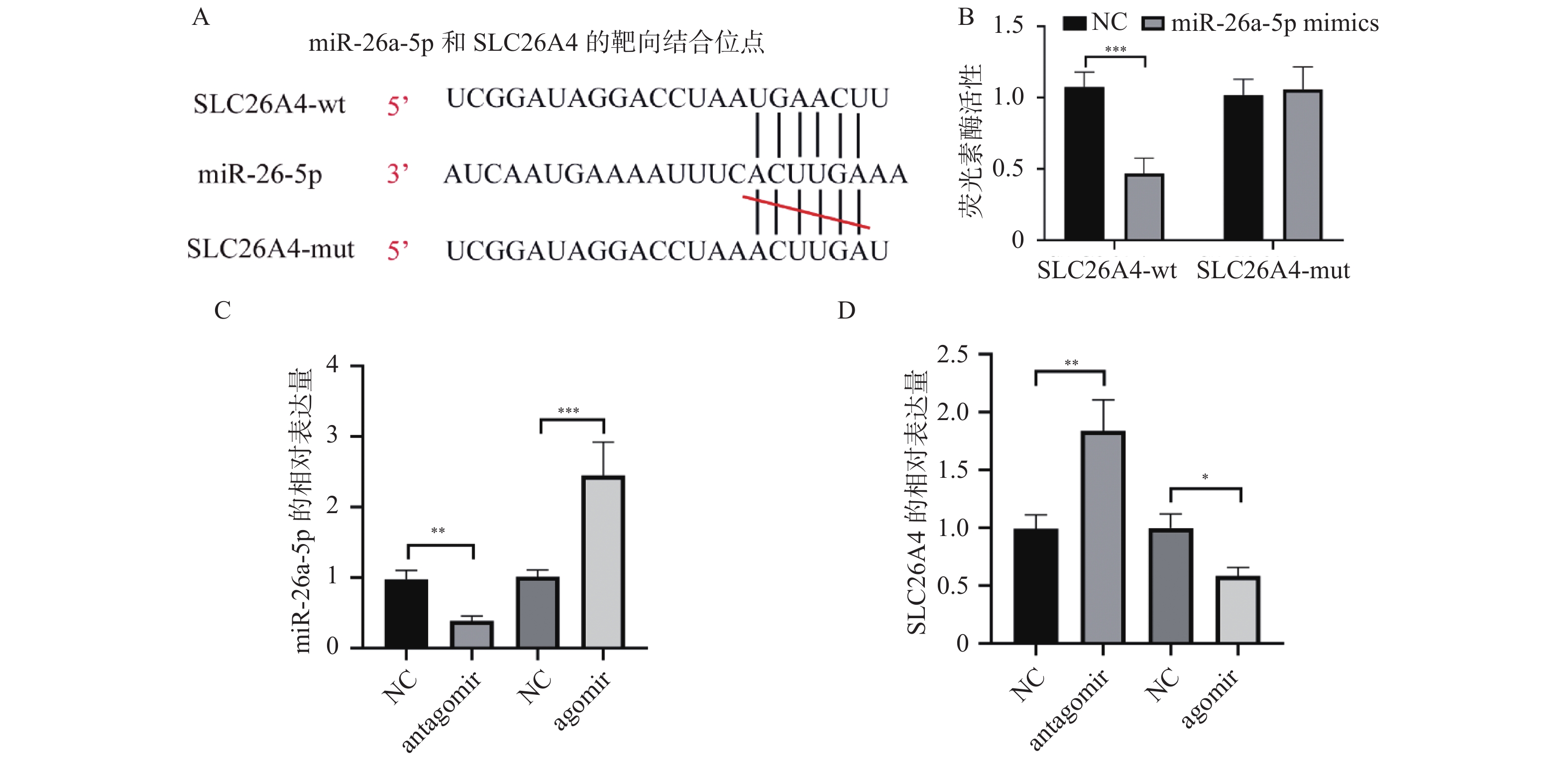
2.3 miR-26a-5p可以靶向调控SLC26A4的表达
使用TargetScan网站(https://www.targetscan.org/vert_72/)对miR-26a-5p和SLC26A4的靶向结合位点进行生信预测,结果显示miR-26a-5p和SLC26A4可以靶向结合,结合位点共有7对碱基,见图3A。通过荧光素酶报告基因实验来检测miR-26a-5p和SLC26A4两者之间是否靶向结合,可以看到野生型SLC26A4组荧光素酶活性与对照组相比明显降低,而突变型SLC26A4无明显差异,见图3B。对C57小鼠分别注射miR-26a-5p的antagomir和agomir,而后对小鼠的耳蜗使用qPCR实验检测miR-26a-5p和SLC26A4的表达量,结果显示,miR-26a-5p的antagomir 可以显著降低miR-26a-5p的表达,而SLC26A4正好相反;此外agomir明显提高了miR-26a-5p的表达,同时也抑制了SLC26A4的表达,见图3C和图3D。
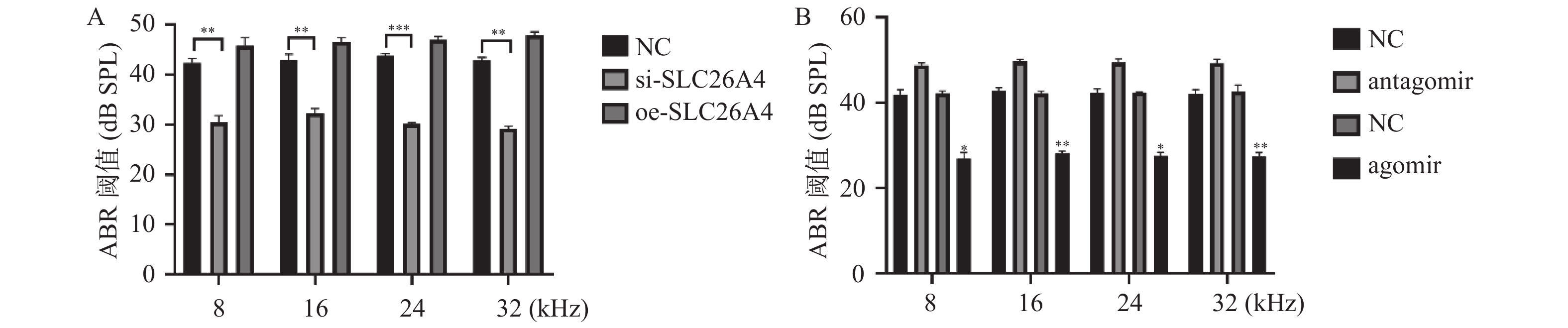
2.4 miR-26a-5p调控SLC26A4改善了听力减退
通过分别给听力损伤小鼠注射miR-26a-5p的干扰和过表达载体以及SLC26A4的干扰和过表达载体,继而使用ABR监测小鼠的听力阈值情况,结果显示,si-SLC26A4和miR-26a-5p agomir可以显著降低小鼠的听力阈值,见图4A和图4B,这表明miR-26a-5p可以通过调控SLC26A4达到改善小鼠听力减退的功能。
3. 讨论
遗传性耳聋在出生缺陷中已经是一种非常常见的临床现状,许多与耳聋(听力损伤)相关的基因已经被陆续确定[7],这就表明致病基因的检测在未来先天性耳聋患者中进行医学检查是不可或缺的[8]。第一个miRNA在1993年被发现, miRNA可以结合到mRNA上,导致靶mRNA完全降解,从而使得靶mRNA的翻译受到抑制[9-10]。miRNAs已被证实广泛存在于不同的细胞和组织中,在细胞周期、细胞凋亡、肿瘤发生和神经发生等生物学过程中发挥重要作用[11],同样在遗传性耳聋中也担任重要的角色[12]。有许多研究表明miRNA的异常导致了耳聋的发生,如Mohammad-Reza发现miR-183的异常会导致毛细胞逐渐失去顶端结构,听力阈值增加,而通过提高miR-183家族在听细胞中的水平,可以恢复耳尖结构和听力[13]。而关于miR-26a-5p与耳聋的机制研究非常少,这就值得笔者去探究miR-26a-5p在耳聋中是什么样的作用。本研究发现miR-26a-5p在小鼠听力损伤后尤其是耳聋后表达异常的降低,在小鼠体内增加其表达后发现小鼠听力阈值变得更高,听力损伤加重,这些结果都说明miR-26a-5p是耳聋的潜在保护基因。
SLC26A4是溶质载体家族的一员,是一种pendrin蛋白编码基因,主要在甲状腺、内耳和肾脏中表达[14]。已有许多研究表明该基因的致病性变异可导致耳聋伴前庭导尿管增大(EVA),包括感音神经性听力损失[15-16]。本次研究发现了SLC26A4在小鼠听力损伤后表达异常高,且miR-26a-5p可以靶向调节SLC26A4的相关表达。在小鼠体内提高miR-26a-5p的表达后,SLC26A4表达明显受到抑制,此外笔者发现小鼠的听力减退得到了改善。同时笔者在小鼠体内抑制掉SLC26A4表达,结果与上面一致,这表明SLC26A4是耳聋的致病基因,会引起一系列的听力损伤。
综上所述,miR-26a-5p 可以通过调控SLC26A4从而挽救听力减退,SLC26A4是耳聋中导致听力减退的罪魁祸首之一,而通过miR-26a-5p吸附SLC26A4靶向抑制其表达后在小鼠动物实验中可以有效改善听力损伤。本次研究从miRNA调控靶mRNA表达机制研究,揭示了miR-26a-5p 调控SLC26A4的作用机制,为miR-26a-5p 和SLC26A4在遗传性耳聋的基因治疗中提供一定的科学依据。
-
表 1 引物序列
Table 1. Primer sequence
基因名称 上游引物序列(5′- 3′) 下游引物序列(5′- 3′) β-actin GTGGGGCGCCCCAGGCACCA CTCCTTAATGTCACGCACGATTT SLC26A4 GCATCCTCTCCATTATCTACA TCCTTAACAGCCATACAGAC miR-26a-5p 通用引物 AGCCAGCGTTCAAGTAATCCAG U6 通用引物 ATGGACTATCATATGCTTACCGTA -
[1] 杨慧波,虞闰六,缪勤,等. 新生儿耳聋基因检测结果分析[J]. 昆明医科大学学报,2020,41(7):144-148. doi: 10.3969/j.issn.1003-4706.2020.07.029 [2] Harris C,Hemer S,Chur-Hansen A. Emotion as motivator: Parents,professionals and diagnosing childhood deafness[J]. Medical Anthropology,2021,40(3):254-266. doi: 10.1080/01459740.2020.1796659 [3] Aifa-Hmani M,Ayadi H. Deafness genes and the mechanism of hearing[J]. Archives de L'institut Pasteur de Tunis,2000,77(1-4):17-21. [4] Knipper M,Van Dijk P,Schulze H,et al. The neural bases of tinnitus:lessons from deafness and cochlear implants[J]. Journal of Neuroscience,2020,40(38):7190-7202. doi: 10.1523/JNEUROSCI.1314-19.2020 [5] 涂云贵,杜琼,余映辉,等. 云南省安宁地区184例耳聋基因筛查的结果[J]. 昆明医科大学学报,2019,40(12):44-48. doi: 10.3969/j.issn.1003-4706.2019.12.010 [6] 郝帅, 于飞, 杨博, 等. D-半乳糖对仔鼠脂质过氧化、听力和耳蜗毛细胞的损伤及其机制. 吉林大学学报(医学版), 2012, 38(3): 419-422. [7] Romano D R,Hashino E,Nelson R F. Deafness-in-a-dish: modeling hereditary deafness with inner ear organoids[J]. Human Genetics,2022,141(3-4):347-362. doi: 10.1007/s00439-021-02325-9 [8] Hardelin J P,Denoyelle F,Levilliers J,et al. Hereditary deafness: Molecular genetics[J]. Medecine Sciences:M/S,2004,20(3):311-316. doi: 10.1051/medsci/2004203311 [9] Ho P T B,Clark I M,Le L T T. MicroRNA-based diagnosis and therapy[J]. International Journal of Molecular Sciences,2022,23(13):7167. doi: 10.3390/ijms23137167 [10] Krol J,Loedige I,Filipowicz W. The widespread regulation of microRNA biogenesis,function and decay[J]. Nature reviews genetics,2010,11(9):597-610. doi: 10.1038/nrg2843 [11] Pritchard C C,Cheng H H,Tewari M. MicroRNA profiling: approaches and considerations[J]. Nature Reviews Genetics,2012,13(5):358-369. doi: 10.1038/nrg3198 [12] Friedman L M,Avraham K B. MicroRNAs and epigenetic regulation in the mammalian inner ear: implications for deafness[J]. Mammalian Genome,2009,20(9-10):581-603. doi: 10.1007/s00335-009-9230-5 [13] Mahmoodian-Sani M R,Mehri-Ghahfarrokhi A. The potential of miR-183 family expression in inner ear for regeneration,treatment,diagnosis and prognosis of hearing loss[J]. Journal of Otology,2017,12(2):55-61. doi: 10.1016/j.joto.2017.03.003 [14] Tawalbeh M,Aburizeg D,Abu Alragheb B O,et al. SLC26A4 phenotypic variability influences intra- and inter-familial diagnosis and management[J]. Genes,2022,13(12):2192. doi: 10.3390/genes13122192 [15] Hilgert N,Smith R J H,Van Camp G. Forty-six genes causing nonsyndromic hearing impairment: which ones should be analyzed in DNA diagnostics?[J]. Mutation Research/Reviews in Mutation Research,2009,681(2-3):189-196. doi: 10.1016/j.mrrev.2008.08.002 [16] Park H J,Shaukat S,Liu X Z,et al. Origins and frequencies of SLC26A4 (PDS) mutations in east and south Asians:global implications for the epidemiology of deafness[J]. Journal of Medical Genetics,2003,40(4):242-248. doi: 10.1136/jmg.40.4.242 期刊类型引用(1)
1. 云丽媛,王竣凤,郭宁,丁瑞美,李海朋,张雪萍. GM1注射液联合巴曲酶治疗突发性耳聋患者的疗效及安全性. 昆明医科大学学报. 2024(06): 140-144 .  本站查看
本站查看其他类型引用(0)
-






 下载:
下载:
 下载:
下载:

