Prospects of Antimicrobial Peptides as Immunomodulators in the Treatment of Bacterial Infections
-
摘要: 自1928年青霉素被用于治疗细菌感染性疾病以来,抗生素拯救了无数患者的生命。然而,随着细菌抗药性的增加,细菌感染性疾病的临床治疗变得愈发困难。抗菌肽因其功能广泛且毒副作用低等特点,被认为是理想的传统抗生素替代品。其中,一些具有免疫调节功能的抗菌肽能够调节机体自身的固有免疫和获得性免疫反应,直接靶向机体免疫系统而非病原微生物,从而避免对病原微生物的选择压力,不易产生耐药性,在抗感染治疗方面具有广阔的应用价值。将从抗菌肽的免疫调节活性、临床治疗中的应用潜力以及局限性等方面进行概述,为新型抗生素的开发利用提供新的视野。Abstract: Since penicillin was used to treat bacterial infectious diseases in 1928, antibiotics have saved countless patients’ lives. However, with the increase of bacterial drug resistance, the clinical treatment of bacterial infectious diseases has become increasingly difficult. Antimicrobial peptides is considered as an ideal substitute for traditional antibiotics because of its wide range of functions and low toxicity. Among them, some antimicrobial peptides with immune regulation function can regulate the innate immunity and acquired immune response of the body, directly target the immune system of the body rather than pathogenic microorganisms, thus avoiding the selection pressure on pathogenic microorganisms, and are not easily resistant to antibiotics. They have broad application value in infection treatment. This article reviews the immunomodulatory activity of antimicrobial peptides, its potential application in clinical treatment, and its limitations, so as to provide a new perspective for the development and utilization of new antibiotics.
-
Key words:
- Bacterial infection /
- Antimicrobial peptides /
- Immune regulation
-
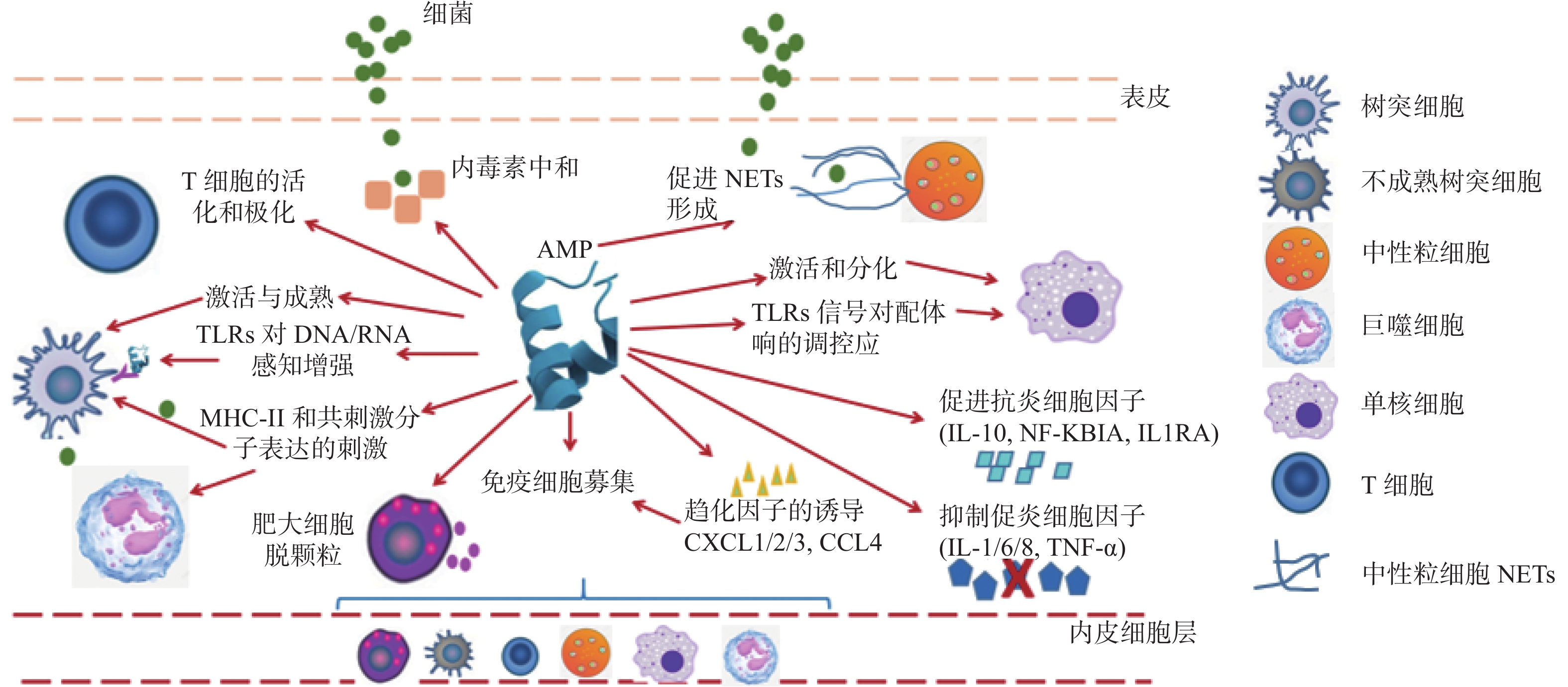
抗菌肽在先天宿主免疫调节的背景下已被研究了40多年。初步研究的重点是了解抗菌肽的抗菌机制,确定其对抗菌功能至关重要的共同结构和理化特征[1]。在过去10多年中,人们在阐明抗菌肽如何与宿主固有免疫和适应性免疫系统相互作用方面取得了很大进展[2]。抗菌肽可以利用宿主自身的免疫系统,抑制潜在的有害炎症反应[3]。同时许多研究表明,抗菌肽可以通过调节宿主固有免疫和适应性免疫来清除病原体[4-7]。
抗菌肽(antimicrobial peptides,AMPs),也称为宿主防御肽(host defense peptides,HDPs),是一种通常带正电荷的短肽,存在于多种生命形式中。在一定条件下,大部分阳离子抗菌肽在体外都具有抗菌作用。然而,在宿主体内,许多阳离子抗菌肽的直接抗菌活性往往被一定浓度的肽抑制和/或被生理浓度的单价和二价阳离子、血清和阴离子大分子如糖胺聚糖所拮抗[8]。因此,阳离子抗菌肽在体内实际上可能并不是通过直接杀死微生物来增强宿主的防御作用,而是通过选择性地增强或调节宿主自身免疫机制,以促进宿主对微生物感染的保护[9]。AMPs在体内的抗菌作用也不能完全被忽略,如:AMPs通常与中性粒细胞胞外陷阱(neutrophil extracellular traps,NETs)相关,并有助于其形成。NETs是中性粒细胞活化后在炎症部位释放的由DNA和蛋白质组成的网状结构,其主要功能是捕获和杀死病原体[10-11]。
截止到目前,AMPs已经被报道具有多种免疫调节活性。如首次报道的AMPs免疫调节活性与其可以趋化白细胞的能力有关[12]。自此之后,AMPs的许多其他免疫调节活性陆续被报道,包括增加趋化因子的表达,调节抗炎细胞因子、促炎细胞因子、活性氧(reactive oxygen species,ROS)、一氧化氮合酶(nitric oxide synthase,NOS)的产生,促进伤口愈合,诱导血管生成,促进白细胞的激活和分化,抗原呈递和适应性免疫的调节等[13-16]。此外,一些AMPs还可以通过结合蛋白质分子以及靶向固有免疫受体来发挥免疫调节活性[2,17]。因此,了解AMPs如何通过免疫调节活性来治疗细菌感染,平衡炎症以及促进免疫稳态,对AMPs的开发具有重要意义。
1. 抗菌肽的免疫调节活性
1.1 白细胞的免疫趋化
AMPs调节免疫系统的重要机制之一是对免疫细胞表现出直接的趋化活性,以及通过诱导趋化因子释放来增强免疫细胞的募集[18]。接下来笔者将对AMPs的直接趋化活性,以及间接趋化活性进行概述。
1.1.1 直接趋化活性
来自不同物种的大量AMPs已被证明可作为固有免疫细胞和适应性免疫细胞的趋化剂[19]。人cathelicidin LL-37被认为是具有多种免疫调节活性的AMPs。 LL-37可以通过甲酰肽受体1(recombinant formyl peptide receptor,FPRL1)吸引中性粒细胞,嗜酸性粒细胞,单核细胞,CD4+ T细胞到微生物入侵的部位,从而激活先天性和适应性免疫系统[20-21]。此外,LL-37还可以通过G蛋白偶联受体吸引肥大细胞到细菌感染部位[22]。然而LL-37不会吸引单核细胞来源的未成熟树突状细胞,因为它们在分化过程中下调了FPRL1[23]。此外,在耐药菌感染的小鼠模型中,合成肽IDR-1通过FPRL1化学吸引中性粒细胞来起到预防和治疗小鼠耐药菌感染的目的[24-25]。
1.1.2 间接趋化活性
AMPs 可以通过刺激趋化因子和趋化因子受体的表达来发挥间接趋化功能。如:LL37可以通过调节趋化因子的分泌来发挥其间接趋化活性。LL37能够单独诱导CXCL8/ IL8的转录,并且可以与TNF-ɑ协同介导CXCL8/IL8的表达[26],而CXCL8/ IL8可以刺激中性粒细胞的迁移。LL37还可以通过诱导CXCL10/IP10的表达来募集巨噬细胞、T细胞、NK细胞和树突状细胞[27]。MCP-1/CCL-2也可以被LL37刺激后以剂量依赖的方式分泌,并且MCP-1可以吸引单核细胞,T细胞和树突状细胞到达细菌感染部位[25]。CCL5可以被LL37上调来增强嗜酸性粒细胞、嗜碱性粒细胞和T细胞的趋化性[28]。除此之外,CXCL1/KC、CCL7/ MCP-3、CCL4、CCL20/MIP3a的表达都可以被 LL-37上调[29]。LL37还可以通过刺激趋化因子受体来发挥趋化功能,50 µg/mL LL-37可以上调小鼠巨噬细胞趋化因子受体的表达,如IL-8受体、CXCR4、CCR2和LFA-1[30]。
1.2 抗原呈递和适应性免疫的调节
除了具有调节固有免疫效应功能外,AMPs还可以作为固有免疫和适应性免疫之间的桥梁,因为其可以将抗原呈递细胞(antigen-presenting cells,APCs)如树突状细胞和单核细胞/巨噬细胞募集到感染部位。树突状细胞是独特有效的前哨白细胞,可以捕获周围组织中的抗原,然后启动和协调原代Th细胞反应[31]。这一过程对于成功防御非自身有害微生物是至关重要的,并且取决于树突状细胞对抗原捕获和呈递能力[32]。LL-37可促进循环造血前体细胞和前体树突细胞向树突状细胞分化,并通过与未成熟树状细胞相互作用影响适应性免疫。LL-37是树突状细胞分化和激活过程的调节剂,可以上调未成熟树状细胞的吞噬能力,调节吞噬受体的表达,上调共刺激分子的表达,促进Th1诱导因子的分泌,并增强Th1反应[33]。除此之外,LL37可以通过活化滤泡树突细胞来增强B细胞的激活/增殖[34]。
1.3 脱颗粒
AMPs可以刺激免疫细胞脱颗粒,然后释放大量促炎和抗菌物质,包括更多AMPs。LL-37 通过G蛋白偶联受体诱导肥大细胞持续的钙动员以及大量的脱颗粒[35-36],这会导致组胺和前列腺素D2(prostaglandin D2,PGD2)的释放[37]。PGD2在炎症后期,可以通过调节促炎细胞因子与抗炎细胞因子的平衡来调节炎症[38]。同时,TLR2配体可以修饰被LL-37激活的肥大细胞,并且肥大细胞的功能由过敏反应转变为固有免疫反应[39]。这可能暗示了在感染的情况下LL37会抑制肥大细胞的过敏反应。
1.4 活性氧(ROS)和活性氮(RNS)产生的调节
一氧化氮(nitric oxide,NO)和ROS具有消除入侵病原体的能力,并且在固有免疫中发挥重要作用[40]。在吞噬细胞内,AMPs可以增强中性粒细胞的呼吸爆发并增加ROS的释放,该过程由黄素酶介导,并通过细胞内Ca2+浓度的增加来实现[41]。除此之外,AMPs能以剂量依赖性的方式中和被内毒素诱导的巨噬细胞中NO和TNF-α的释放[14]。
1.5 炎症反应的调节
如前所述,AMPs具有激活免疫系统的多种功能,可以将其归类为有助于清除病原体的促炎反应。现在有相当多的证据表明,AMPs可以改变炎症反应的性质,增强了传统上被认为是促炎的某些活性,同时抑制了微生物的特异性分子对促炎细胞因子的有效诱导[42-43]。AMPs的主要特征可能是细菌特异性诱导炎症后调节炎症,并有助于过渡到更平衡的炎症反应中[44]。因此,将它们描述为促炎或抗炎分子,不如将AMPs定义为可以促进免疫稳态的分子[45]。AMPs还可以抑制内毒素和其他TLR激动剂诱导的促炎反应[46],如:LL-37可以通过以下方式抑制LPS诱导的人巨噬细胞中的促炎反应:(1)直接与LPS相互作用以减少其与LPS结合蛋白(lipopolysaccharide bindingprotein,LBP),淋巴细胞抗原96(lymphocyte antigen 96,LY96)或TLR-4受体复合物的结合,从而减少下游途径的激活;(2)抑制LPS诱导的NF-κB中p65、p50的活化,并通过MyD88减少LPS诱导的TREM-1上调[47];(3)通过抑制某些促炎基因,包括NF-κB1(P105/P50)和TNF-α诱导蛋白2(recombinant tumor necrosis factor alpha induced protein 2,TNFαIP2),同时上调抗炎细胞因子,例如:IL-10和TNF-α诱导的蛋白3(recombinant tumor necrosis factor alpha induced protein 3,TNFαIP3)来选择性地调节基因转录;(4)激活MAPK和PI3K途径来影响促炎途径;(5)可能直接或间接影响TNF-α蛋白的翻译,稳定或加工。然而,在特定情况下,LPS和LL37之间的相互作用可以导致细胞激活,如:LL37-LPS复合物在体外被人支气管上皮细胞更有效地识别,随后导致细胞内TLR4活化增强和IL-6产生增加[48];同样,与LPS本身相比,人类腺癌结肠上皮细胞系也对LL37-LPS复合物的炎症反应增强[49]。LL37还可以抑制IL-32诱导的炎性单核细胞产生IL-1β、TNF-α、IL-6[50]。除巨噬细胞外,LL37可以降低LPS导致的人中性粒细胞[51],树突状细胞[52]和B淋巴细胞[53]的促炎细胞因子水平,并且其还可以抑制LTA诱导的外周血单个核细胞[27]和树突状细胞[52]中促炎细胞因子TNF、IL-6的释放。
综上所述,AMPs的免疫调节活性,见图1。
2. 临床治疗的应用和挑战
随着细菌对常规抗生素耐药性的增加,以及缺乏针对耐药菌感染的新疗法[54-55]。人们越来越关注利用AMPs的免疫调节活性来靶向治疗多重耐药菌。许多具有抗菌和/或免疫调节特性的肽已在临床上研究了对多重耐药菌的疗效。然而,到目前为止,只有少数AMPs衍生的化合物进入了临床,并且大多数临床试验侧重于局部而不是系统应用[56]。除此之外,AMPs的临床应用还与很多因素相关,例如给药途径、稳定性、生物利用度、毒性、免疫原性,还与它们昂贵的生产成本有关[57-58]。同时,已有报道显示AMPs可以对细菌产生耐药性[59]。然而,对于具有免疫调节活性的AMPs,其针对的是免疫系统而不是病原体,所以免疫调节肽可以避免细菌耐药性的问题[54]。
为了使AMPs在临床上有更好的应用,有以下几种方法可以克服AMPs的蛋白水解敏感性,并改善其稳定性。在化学修饰方面,异构化即用D-氨基酸替换一种或多种L-氨基酸[60];环化即通过引入二硫键或连接N和C末端来实现[61];脂化即将一个或多个脂肪酸链连接到AMPs的N末端或赖氨酸残基的氨基上[62]。除此之外,已经开发了多种递送系统或纳米载体,以提高AMPs的稳定性和生物利用度,并且可以将其递送到临床关注的特定部位,以可控的方式释放它们[63]。研究最多的AMPs递送系统包括脂质体制剂,聚合物纳米结构,透明质酸纳米凝胶,DNA“笼子”,聚乳酸羟基乙酸共聚物和介孔二氧化硅纳米微球等[64-68]。AMPs还可以与生物聚合物(例如壳聚糖和透明质酸)结合,这些生物聚合物具有多个用于连接肽的官能团,来增加其生物相容性以及抗菌活性[69]。
3. 小结
抗生素耐药性的增加以及缺乏新的治疗耐药菌感染的方法,这使得耐药菌感染严重威胁着人类的生命健康安全。而抗菌肽是一种独特的分子,有望成为治疗多重耐药菌感染的生物候选者。现在AMPs已经被证明在机体免疫调节中起着至关重要的作用,如:固有免疫的激活,抗原呈递和吞噬作用的增强,影响适应性免疫以及强大的抗炎功能。因此,利用AMPs的免疫调节活性来治疗耐药菌感染被认为是一种新的抗感染治疗方法。此外,正在开发许多新方法来改善AMPs的生物相容性、稳定性以及毒性等。使用这些新方法可以改善AMPs的局限性,并考虑到现在有大量可用的肽序列,所以可以合理的预测AMPs将成为未来治疗多重耐药病原体感染的有效药物。
-
[1] Zasloff M. Antimicrobial peptides of multicellular organisms[J]. Nature,2002,415(6870):389-395. doi: 10.1038/415389a [2] Hancock R E,Nijnik A,Philpott D J. Modulating immunity as a therapy for bacterial infections[J]. Nat Rev Microbiol,2012,10(4):243-254. doi: 10.1038/nrmicro2745 [3] Hamill P,Brown K,Jenssen H,et al. Novel anti-infectives: Is host defence the answer?[J]. Curr Opin Biotechnol,2008,19(6):628-636. doi: 10.1016/j.copbio.2008.10.006 [4] Blyth G a D,Connors L,Fodor C,et al. The network of colonic host defense peptides as an innate immune defense against enteropathogenic bacteria[J]. Front Immunol,2020,11(15):965. [5] Shelley J R,Davidson D J,Dorin J R. The dichotomous responses driven by β-defensins[J]. Front Immunol,2020,11(56):1176. [6] Wu D,Fu L,Wen W,et al. The dual antimicrobial and immunomodulatory roles of host defense peptides and their applications in animal production[J]. Anim Sci Biotechnol,2022,13(1):141. doi: 10.1186/s40104-022-00796-y [7] Buccini D F,Cardoso M H,Franco O L. Antimicrobial peptides and cell-penetrating peptides for treating intracellular bacterial infections[J]. Front Cell Infect Microbiol,2021,10:612931. doi: 10.3389/fcimb.2020.612931 [8] Bowdish D M,Davidson D J,Lau Y E,et al. Impact of LL-37 on anti-infective immunity[J]. J Leukoc Biol,2005,77(4):451-459. [9] Brown K L,Hancock R E. Cationic host defense (antimicrobial) peptides[J]. Curr Opin Immunol,2006,18(1):24-30. doi: 10.1016/j.coi.2005.11.004 [10] Döring Y,Libby P,Soehnlein O. Neutrophil extracellular traps participate in cardiovascular diseases:Recent experimental and clinical insights[J]. Circ Res,2020,126(9):1228-1241. doi: 10.1161/CIRCRESAHA.120.315931 [11] Alford M A,Baquir B,Santana F L,et al. Cathelicidin host defense peptides and inflammatory signaling:Striking a balance[J]. Front Microbiol,2020,11:1902. doi: 10.3389/fmicb.2020.01902 [12] Territo M C,Ganz T,Selsted M E,et al. Monocyte-chemotactic activity of defensins from human neutrophils[J]. J Clin Invest,1989,84(6):2017-2020. doi: 10.1172/JCI114394 [13] Hölzl M A,Hofer J,Steinberger P,et al. Host antimicrobial proteins as endogenous immunomodulators[J]. Immunol Lett,2008,119(1-2):4-11. doi: 10.1016/j.imlet.2008.05.003 [14] Van Harten R M,Van Woudenbergh E,Van Dijk A,et al. Cathelicidins: Immunomodulatory antimicrobials[J]. Vaccines (Basel),2018,6(3):1-23. [15] Zughaier S M,Shafer W M,Stephens D S. Antimicrobial peptides and endotoxin inhibit cytokine and nitric oxide release but amplify respiratory burst response in human and murine macrophages[J]. Cell Microbiol,2005,7(9):1251-1262. doi: 10.1111/j.1462-5822.2005.00549.x [16] Cai J,Cui X,Wang X,et al. A Novel anti-infective peptide BCCY-1 with immunomodulatory activities[J]. Front Immunol,2021,12:713960. doi: 10.3389/fimmu.2021.713960 [17] Laman A G,Lathe R,Savinov G V,et al. Innate immunity: Bacterial cell-wall muramyl peptide targets the conserved transcription factor YB-1[J]. FEBS Lett,2015,589(15):1819-1824. doi: 10.1016/j.febslet.2015.05.028 [18] Mookherjee N,Hancock R E. Cationic host defence peptides: Innate immune regulatory peptides as a novel approach for treating infections[J]. Cell Mol Life Sci,2007,64(7-8):922-933. doi: 10.1007/s00018-007-6475-6 [19] Nijnik A,Hancock R. Host defence peptides: Antimicrobial and immunomodulatory activity and potential applications for tackling antibiotic-resistant infections[J]. Emerg Health Threats J,2009,2:e1. [20] Tjabringa G S,Ninaber D K,Drijfhout J W,et al. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors[J]. Int Arch Allergy Immunol,2006,140(2):103-112. doi: 10.1159/000092305 [21] De Y,Chen Q,Schmidt A P,et al. LL-37,the neutrophil granule and epithelial cell-derived cathelicidin,utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils,monocytes,and T cells[J]. J Exp Med,2000,192(7):1069-1074. doi: 10.1084/jem.192.7.1069 [22] Niyonsaba F,Iwabuchi K,Someya A,et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis[J]. Immunology,2002,106(1):20-26. doi: 10.1046/j.1365-2567.2002.01398.x [23] Yang D,Chen Q,Le Y,et al. Differential regulation of formyl peptide receptor-like 1 expression during the differentiation of monocytes to dendritic cells and macrophages[J]. J Immunol,2001,166(6):4092-4098. doi: 10.4049/jimmunol.166.6.4092 [24] Lee H Y,Bae Y S. The anti-infective peptide,innate defense-regulator peptide,stimulates neutrophil chemotaxis via a formyl peptide receptor[J]. Biochem Biophys Res Commun,2008,369(2):573-578. doi: 10.1016/j.bbrc.2008.02.046 [25] Scott M G,Dullaghan E,Mookherjee N,et al. An anti-infective peptide that selectively modulates the innate immune response[J]. Nat Biotechnol,2007,25(4):465-472. doi: 10.1038/nbt1288 [26] Chen X,Takai T,Xie Yet al. Human antimicrobial peptide LL-37 modulates proinflammatory responses induced by cytokine milieus and double-stranded RNA in human keratinocytes[J]. Biochem Biophys Res Commun,2013,433(4):532-537. doi: 10.1016/j.bbrc.2013.03.024 [27] Coorens M,Scheenstra M R,Veldhuizen E J,et al. Interspecies cathelicidin comparison reveals divergence in antimicrobial activity,TLR modulation,chemokine induction and regulation of phagocytosis[J]. Sci Rep,2017,7:40874. doi: 10.1038/srep40874 [28] Bautista-Hernández L A,Gómez-Olivares J L,Buentello-Volante B,et al. Fibroblasts: The unknown sentinels eliciting immune responses against microorganisms[J]. Eur J Microbiol Immunol (Bp),2017,7(3):151-157. doi: 10.1556/1886.2017.00009 [29] Li N,Yamasaki K,Saito R,et al. Alarmin function of cathelicidin antimicrobial peptide LL37 through IL-36γ induction in human epidermal keratinocytes[J]. J Immunol,2014,193(10):5140-5148. [30] Scott M G,Davidson D J,Gold M R,et al. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses[J]. J Immunol,2002,169(7):3883-3891. [31] Moser M,Murphy K M. Dendritic cell regulation of TH1-TH2 development[J]. Nat Immunol,2000,1(3):199-205. doi: 10.1038/79734 [32] Davidson D J,Currie A J,Reid G S,et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization[J]. J Immunol,2004,172(2):1146-1156. doi: 10.4049/jimmunol.172.2.1146 [33] Bandholtz L,Ekman G J,Vilhelmsson M,et al. Antimicrobial peptide LL-37 internalized by immature human dendritic cells alters their phenotype[J]. Scand J Immunol,2006,63(6):410-419. doi: 10.1111/j.1365-3083.2006.001752.x [34] Kim S H,Kim Y N,Jang Y S. Cutting Edge: LL-37-mediated formyl peptide receptor-2 signaling in follicular dendritic cells contributes to B cell activation in peyer's patch germinal centers[J]. J Immunol,2017,198(2):629-633. doi: 10.4049/jimmunol.1600886 [35] Yu Y,Zhang Y,Zhang Y,et al. LL-37-induced human mast cell activation through G protein-coupled receptor MrgX2[J]. Int Immunopharmacol,2017,49:6-12. doi: 10.1016/j.intimp.2017.05.016 [36] Gupta K,Subramanian H,Ali H. Modulation of host defense peptide-mediated human mast cell activation by LPS[J]. Innate Immun,2016,22(1):21-30. doi: 10.1177/1753425915610643 [37] Subramanian H,Gupta K,Guo Q,et al. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: Resistance to receptor phosphorylation,desensitization,and internalization[J]. J Biol Chem,2011,286(52):44739-44749. doi: 10.1074/jbc.M111.277152 [38] Rajakariar R,Hilliard M,Lawrence T,et al. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2[J]. Proc Natl Acad Sci U S A,2007,104(52):20979-20984. doi: 10.1073/pnas.0707394104 [39] Zhang Y Y,Yu Y Y,Zhang Y R,et al. The modulatory effect of TLR2 on LL-37-induced human mast cells activation[J]. Biochem Biophys Res Commun,2016,470(2):368-374. doi: 10.1016/j.bbrc.2016.01.037 [40] Andrés C M C,Pérez De La Lastra J M,Juan C A,et al. The role of reactive species on innate immunity[J]. Vaccines (Basel),2022,10(10):1735. doi: 10.3390/vaccines10101735 [41] Yang B,Good D,Mosaiab T,et al. Significance of LL-37 on immunomodulation and disease outcome[J]. Biomed Res Int,2020,2020:8349712. [42] Yang Y,Jing W,Qiao L,et al. A non-bactericidal cathelicidin provides prophylactic efficacy against bacterial infection by driving phagocyte influx[J]. Elife,2022,11:e72849. doi: 10.7554/eLife.72849 [43] Jinwei C,Xin C,Tiaofei Y,et al. Characterization and functional analysis of cathelicidin-MH,a novel frog-derived peptide with anti-septicemic properties[J]. Elife,2021,10:e64411. doi: 10.7554/eLife.64411 [44] Yeung A T,Gellatly S L,Hancock R E. Multifunctional cationic host defence peptides and their clinical applications[J]. Cell Mol Life Sci,2011,68(13):2161-2176. doi: 10.1007/s00018-011-0710-x [45] Mookherjee N,Anderson M A,Haagsman H P,et al. Antimicrobial host defence peptides: Functions and clinical potential[J]. Nat Rev Drug Discov,2020,19(5):311-332. doi: 10.1038/s41573-019-0058-8 [46] Mookherjee N,Brown K L,Bowdish D M,et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37[J]. J Immunol,2006,176(4):2455-2464. doi: 10.4049/jimmunol.176.4.2455 [47] Amatngalim G D,Nijnik A,Hiemstra P S,et al. Cathelicidin peptide LL-37 modulates TREM-1 expression and inflammatory responses to microbial compounds[J]. Inflammation,2011,34(5):412-425. doi: 10.1007/s10753-010-9248-6 [48] Shaykhiev R,Sierigk J,Herr C,et al. The antimicrobial peptide cathelicidin enhances activation of lung epithelial cells by LPS[J]. Faseb J,2010,24(12):4756-4766. doi: 10.1096/fj.09-151332 [49] Marin M,Holani R,Shah C B,et al. Cathelicidin modulates synthesis of Toll-like Receptors (TLRs) 4 and 9 in colonic epithelium[J]. Mol Immunol,2017,91:249-258. doi: 10.1016/j.molimm.2017.09.011 [50] Choi K Y,Napper S,Mookherjee N. Human cathelicidin LL-37 and its derivative IG-19 regulate interleukin-32-induced inflammation[J]. Immunology,2014,143(1):68-80. doi: 10.1111/imm.12291 [51] Niyonsaba F,Madera L,Afacan N,et al. The innate defense regulator peptides IDR-HH2,IDR-1002,and IDR-1018 modulate human neutrophil functions[J]. J Leukoc Biol,2013,94(1):159-170. doi: 10.1189/jlb.1012497 [52] Kandler K,Shaykhiev R,Kleemann P,et al. The anti-microbial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands[J]. Int Immunol,2006,18(12):1729-1736. doi: 10.1093/intimm/dxl107 [53] Nijnik A,Pistolic J,Wyatt A,et al. Human cathelicidin peptide LL-37 modulates the effects of IFN-gamma on APCs[J]. J Immunol,2009,183(9):5788-5798. doi: 10.4049/jimmunol.0901491 [54] Hilchie A L,Wuerth K,Hancock R E. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides[J]. Nat Chem Biol,2013,9(12):761-768. doi: 10.1038/nchembio.1393 [55] Luo Y,Song Y. Mechanism of antimicrobial peptides: Antimicrobial,anti-Inflammatory and antibiofilm activities[J]. Int J Mol Sci,2021,22(21):11401. doi: 10.3390/ijms222111401 [56] Afacan N J,Yeung A T,Pena O M,et al. Therapeutic potential of host defense peptides in antibiotic-resistant infections[J]. Curr Pharm Des,2012,18(6):807-819. doi: 10.2174/138161212799277617 [57] Vlieghe P,Lisowski V,Martinez J,et al. Synthetic therapeutic peptides: Science and Market[J]. Drug Discov Today,2010,15(1-2):40-56. doi: 10.1016/j.drudis.2009.10.009 [58] Duarte-Mata D I,Salinas-Carmona M C. Antimicrobial peptides’ immune modulation role in intracellular bacterial infection[J]. Front Immunol,2023,14:1119574. doi: 10.3389/fimmu.2023.1119574 [59] Abdi M,Mirkalantari S,Amirmozafari N. Bacterial resistance to antimicrobial peptides[J]. J Pept Sci,2019,25(11):e3210. [60] Jia F,Wang J,Peng J,et al. D-amino acid substitution enhances the stability of antimicrobial peptide polybia-CP[J]. Acta Biochim Biophys Sin (Shanghai),2017,49(10):916-925. doi: 10.1093/abbs/gmx091 [61] Ting D S J,Beuerman R W,Dua H S,et al. Strategies in translating the therapeutic potentials of host defense peptides[J]. Front Immunol,2020,11:983. doi: 10.3389/fimmu.2020.00983 [62] Rounds T,Straus S K. Lipidation of antimicrobial peptides as a design strategy for future alternatives to antibiotics[J]. Int J Mol Sci,2020,21(24):9692. doi: 10.3390/ijms21249692 [63] Mahlapuu M,Håkansson J,Ringstad L,et al. Antimicrobial peptides: An emerging category of therapeutic agents[J]. Front Cell Infect Microbiol,2016,6:194. [64] Braun K,Pochert A,Lindén M,et al. Membrane interactions of mesoporous silica nanoparticles as carriers of antimicrobial peptides[J]. J Colloid Interface Sci,2016,475:161-170. doi: 10.1016/j.jcis.2016.05.002 [65] Boge L,Bysell H,Ringstad L,et al. Lipid-Based liquid crystals as carriers for antimicrobial peptides:Phase behavior and antimicrobial effect[J]. Langmuir,2016,32(17):4217-4228. doi: 10.1021/acs.langmuir.6b00338 [66] Silva J P,Gonçalves C,Costa C,et al. Delivery of LLKKK18 loaded into self-assembling hyaluronic acid nanogel for tuberculosis treatment[J]. J Control Release,2016,235:112-124. doi: 10.1016/j.jconrel.2016.05.064 [67] D’angelo I,Casciaro B,Miro A,et al. Overcoming barriers in Pseudomonas aeruginosa lung infections: Engineered nanoparticles for local delivery of a cationic antimicrobial peptide[J]. Colloids Surf B Biointerfaces,2015,135:717-725. doi: 10.1016/j.colsurfb.2015.08.027 [68] Sandreschi S,Piras A M,Batoni G,et al. Perspectives on polymeric nanostructures for the therapeutic application of antimicrobial peptides[J]. Nanomedicine (Lond),2016,11(13):1729-1744. doi: 10.2217/nnm-2016-0057 [69] Abd El-Hack M E,El-Saadony M T,Shafi M E,et al. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review[J]. Int J Biol Macromol,2020,164:2726-2744. doi: 10.1016/j.ijbiomac.2020.08.153 期刊类型引用(4)
1. 高书娟. 日粮添加抗菌肽对仔猪生长性能、腹泻率、免疫机体功能及肠道菌群的影响. 中国饲料. 2024(10): 47-50 .  百度学术
百度学术2. 于天明,田玉虎,刘春青,李建喜,王磊,李凤华. 枯草芽孢杆菌源抗菌肽发酵条件优化及其对雏鸡的抗炎作用. 现代畜牧兽医. 2024(06): 19-23 .  百度学术
百度学术3. 樊佳奇,韩雯笑,范慧敏,陈宝江,王宏,郅永伟,崔秋佳,陈赛娟. 酵母肽对肉兔生长性能以及脂多糖应激肉兔血清细胞因子和肝脏抗氧化能力的影响. 动物营养学报. 2024(08): 5308-5318 .  百度学术
百度学术4. 闫丽文,胡祖成,彭凤栖,胡文均,胡海燕,陈基杰,陆洋. 抗菌肽Dermaseptin-PP协同化疗药物抗肿瘤并逆转A549/DDP细胞顺铂耐药性. 中国现代中药. 2024(10): 1727-1738 .  百度学术
百度学术其他类型引用(1)
-






 下载:
下载:
 下载:
下载:

