In Vitro Inhibition of Coxsackievirus by Blumea Balsamifera (L.)DC Extracts
-
摘要:
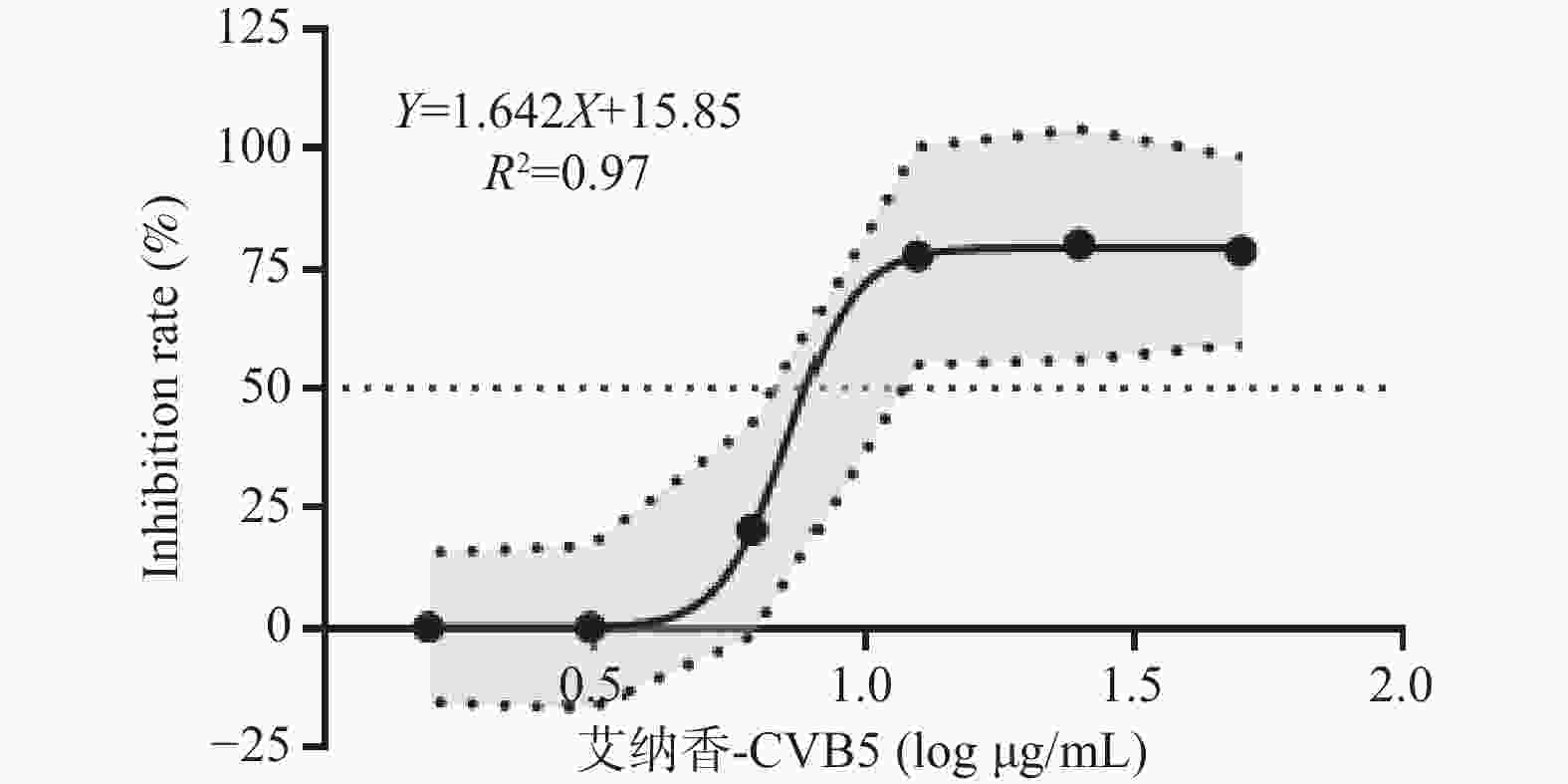
目的 对艾纳香提取物体外抗柯萨奇病毒(CVB5)效果进行研究。 方法 采用倍数稀释的柯萨奇病毒液并将其与RD细胞共同孵育以确定其TCID50值;将不同浓度的艾纳香提取物添加到含有RD细胞的96孔板内,以此评估其对细胞活力的影响程度;在含有RD细胞及艾纳香提取物的96孔板内进一步观察艾纳香提取物对抗柯萨奇病毒的能力。 结果 柯萨奇病毒液的TCID50值为10−7.67。艾纳香提取物对柯萨奇病毒的抑制率随浓度的上升而增大,其针对柯萨奇病毒的IC50值则达到了7.26 mg/L。提取物在50 mg/L的浓度时可导致RD细胞活力降低(P < 0.05),但在6.25~50 mg/L的浓度范围里却能使含有病毒的RD细胞活力上升(P < 0.05),并且选择指数(SI)超过了6.89。 结论 艾纳香提取物具备体外对抗柯萨奇病毒的活性。 Abstract:Objective To investigate the in vitro antiviral effects of Blumea balsamifera(L.)DC. extract against Coxsackievirus B5 (CVB5). Methods A series of dilutions of Coxsackievirus were prepared and co-cultured with RD cells to determine the TCID50 value. Subsequently, different concentrations of the extract were added to a 96-well plate containing RD cells to evaluate their impact on cell viability. The ability of Blumea balsamifera extract to inhibit Coxsackievirus was further observed in the 96-well plate containing RD cells and the extract. Results The TCID50 value of Coxsackie virus solution was 10−7.67. The inhibition rate of Blumea balsamifera extract against Coxsackievirus increased with concentration, with an IC50 value of 7.26 mg/L. At a concentration of 50 mg/L, the extract caused a decrease in RD cell viability(P < 0.05), but within the concentration range of 6.25 to 50 mg/L, it increased the viability of virus-infected RD cells(P < 0.05), with a selectivity index (SI) exceeding 6.89. Conclusion Blumea balsamifera(L.)DC. extract exhibits in vitro activity against Coxsackievirus. -
Key words:
- Blumea balsamifera(L.)DC. /
- Antiviral agents /
- Coxsackie virus
-
表 1 提取物对RD细胞活力的影响($\bar x \pm s $)
Table 1. Impact of extract on the viability of RD cells ($\bar x \pm s $)
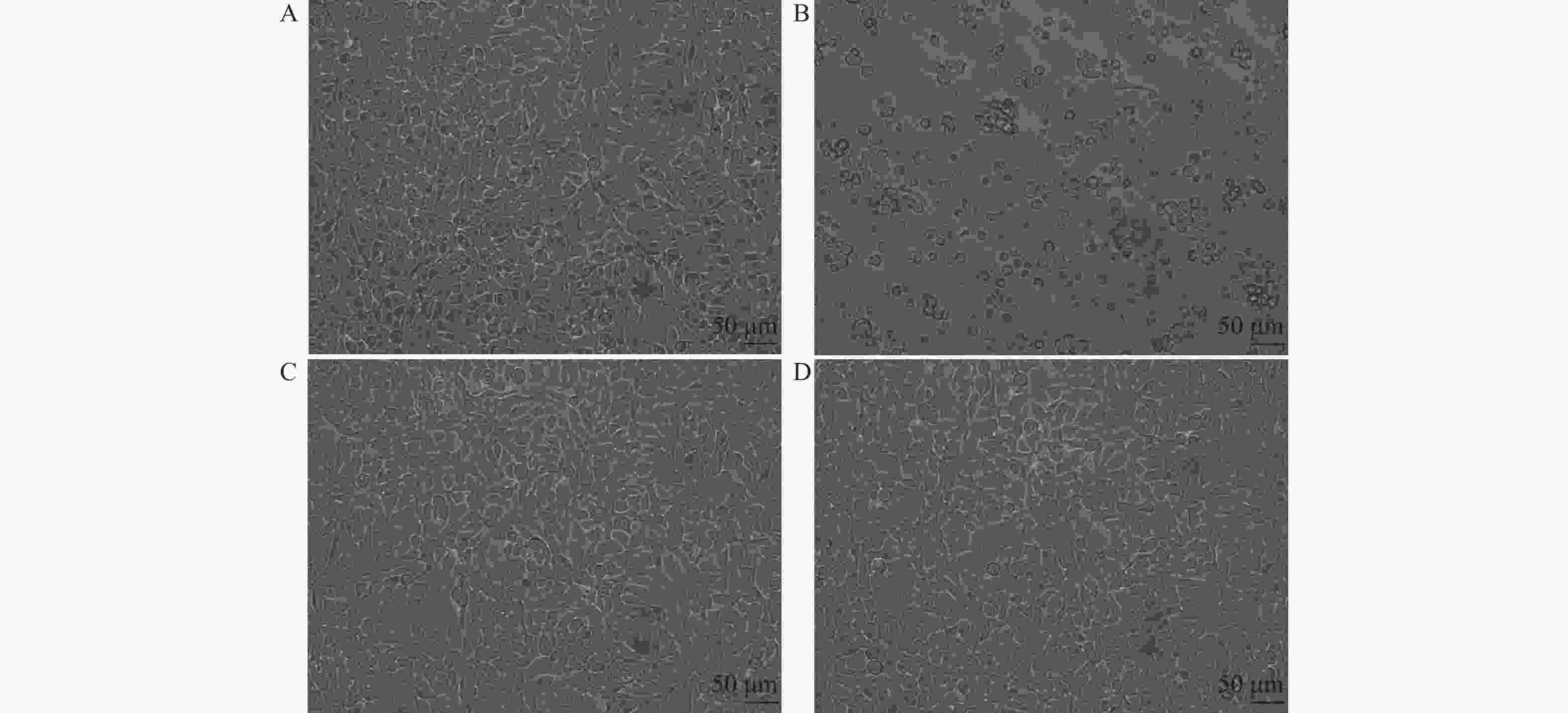
分组 干预剂量 细胞活力(%) P 艾纳香组 50 mg/L 73.39 ± 0.26* 0.0195 25 mg/L 104.49 ± 0.46 P > 0.05 12.5 mg/L 108.28 ± 0.43 P > 0.05 6.25 mg/L 111.12 ± 0.40 P > 0.05 3.13 mg/L 121.40 ± 0.37* P < 0.05 1.56 mg/L 99.20 ± 0.04 P > 0.05 0.78 mg/L 115.27 ± 0.35 P > 0.05 0.39 mg/L 115.17 ± 0.45 P > 0.05 阳性药物组(Flu) 12.5 μM 101.68 ± 0.40 P > 0.05 空白对照组 仅含1%DMSO培养基 100 ± 0.43 与空白对照组比较,* P < 0.05;F(9,19) = 3.241,P = 0.0148 。表 2 提取物对病毒的抑制作用($\bar x \pm s $)
Table 2. Inhibitory effect of extract on virus ($\bar x \pm s $)
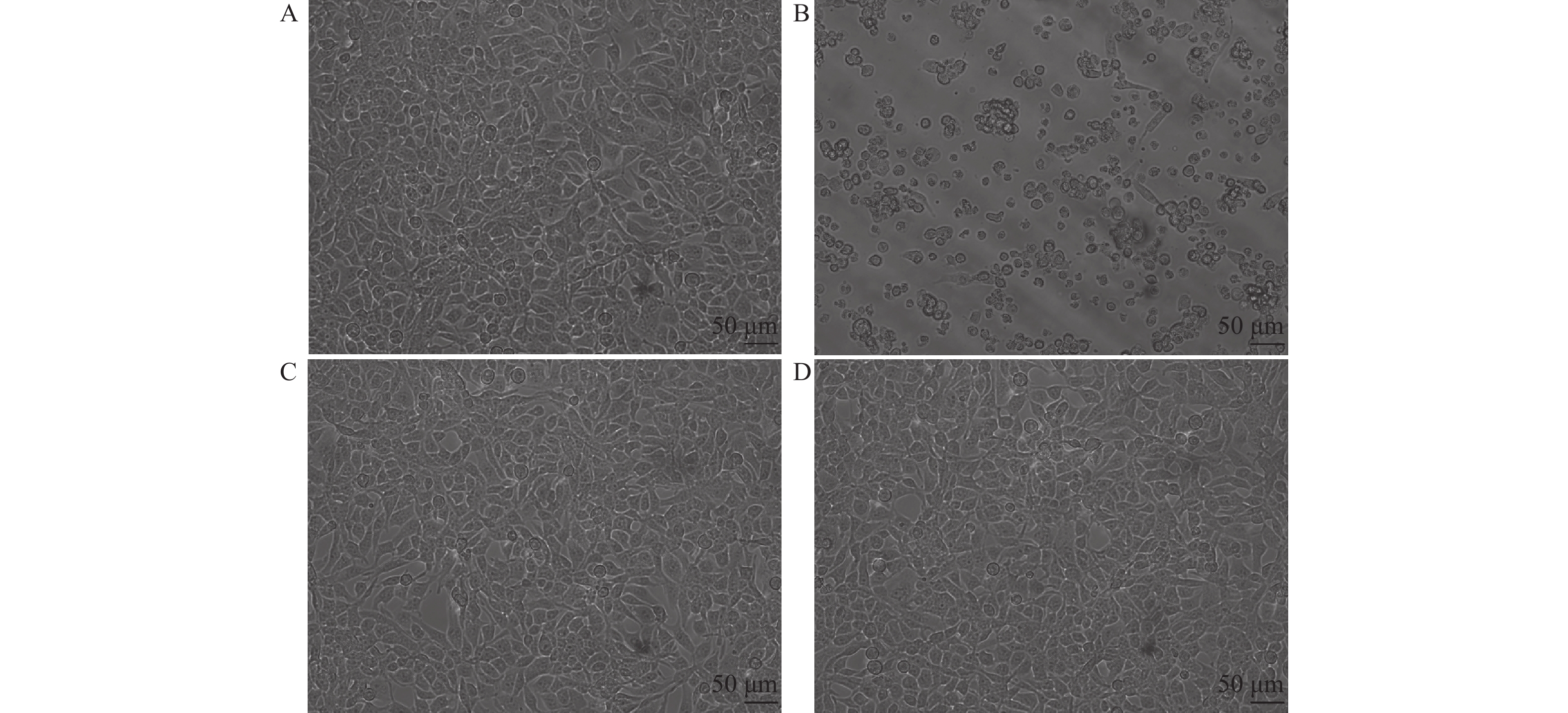
组别 干预剂量 细胞活力(%) 显著性(P) 显著性(P) 病毒抑制率(%) 艾纳香组 50 mg/L 54.12 ± 0.04**## < 0.0001 < 0.0001 78.69 ± 7.90 25 mg/L 86.43 ± 0.10** < 0.0001 0.4707 80.03 ± 9.74 12.5 mg/L 88.04 ± 0.01** < 0.0001 0.6942 77.63 ± 9.20 6.25 mg/L 39.12 ± 0.05**## < 0.0001 < 0.0001 20.41 ± 8.98 3.13 mg/L 8.44 ± 0.01## 0.9997 < 0.0001 0 1.56 mg/L 7.14 ± 0.01## 0.9970 < 0.0001 0 0.78 mg/L 7.98 ± 0.02## 0.9995 < 0.0001 0 0.39 mg/L 8.78 ± 0.01## 0.9998 < 0.0001 0 阳性药物组(Flu) 12.5 μmol/L 94.30 ± 0.07** < 0.0001 91.85 ± 5.81 空白对照组 仅含1%DMSO培养基 9.53 ± 0.02## -- 不加病毒 100 ± 0.43** -- 与加入病毒的空白对照组比较,F(9,20) = 132,*P < 0.05,**P < 0.01;与阳性药物组比较,F(8,18) = 1,#P < 0.05,##P < 0.01。 -
[1] 刘传霞,陈谦明. 儿童口腔黏膜糜烂溃疡类疾病的临床诊断[J]. 浙江大学学报(医学版),2021,50(2):155-161. [2] National Health Commission of the People's Republic of China. 手足口病诊疗指南(2018年版) [J]. 中华临床感染病杂志,2018(3). [3] 中华医学会儿科学分会感染学组,国家感染性疾病医疗质量控制中心. 疱疹性咽峡炎诊断及治疗专家共识(2019年版)[J]. 中华儿科杂志,2019,57(3):4. [4] 郑家喜. 金喉健喷雾剂治疗复发性口腔溃疡近期疗效分析[J]. 中国医药科学,2011,1(13):106-107. [5] 闫文军. 金喉健喷雾剂混合氢氧化钙糊剂用于慢性根尖周炎的临床效果分析[J]. 中国民康医学,2014,26(17):2. doi: 10.3969/j.issn.1672-0369.2014.17.012 [6] 叶煜晟. 金喉健超声雾化剂联合利咽饮治疗小儿急慢性喉炎的效果评价[J]. 中国当代医药,2014,21(14):3. [7] 严淑颜,戴汝均. 康复新液联用金喉健治疗手足口病的口腔疱疹的效果[J]. 中国当代医药,2021,28(17):3. [8] 马颖,宋翠萍,王淑忠. 金喉健喷雾剂联合利巴韦林治疗小儿疱疹性口腔炎疗效观察[J]. 中国煤炭工业医学杂志,2014,17(8):3. [9] 温荣城,贾金艳,李霞,等. 金喉健配方超临界提取工艺产物体外抗病毒研究[J]. 中国民族民间医药,2021,30(3):15-18. [10] 丁健,张先华. 金喉健喷雾剂对小儿急性化脓性扁桃体炎的疗效观察[J]. 湖南师范大学学报(医学版),2008,(2):3. [11] 杜广亮,廖媛. 金喉健喷雾剂辅助治疗小儿疱疹性咽峡炎疗效观察[J]. 中医临床研究,2017,9(32):31-32. doi: 10.3969/j.issn.1674-7860.2017.32.011 [12] 梁建卫,张玉振,张静,等. 清心泻脾汤联合金喉健喷雾剂治疗手足口病普通型100例[J]. 中药材,2012,35(9):3. [13] 柴立,陈真,俞飞,等. 艾纳香提取物在制备抗流感病毒的药物中的新用途 [P]. 贵州: CN202110082036.9,2022-04-12. [14] 柴立,贾金艳,李霞,等. 一种具有抗流感病毒作用的艾纳香提取物及其制备方法 [P]. 贵州: CN202110082021.2,2023-05-12. [15] Shao L,Yang F,Su Y,et al. Design and synthesis of oleanolic acid trimers to enhance inhibition of influenza virus entry[J]. ACS medicinal chemistry letters,2021,12(11):1759-1765. doi: 10.1021/acsmedchemlett.1c00374 [16] Starkey S Y,Mar K,Khaslavsky S,et al. Atypical cutaneous findings of hand-foot-mouth disease in children: A systematic review[J]. Pediatric Dermatology,2024,41(1):23-27. doi: 10.1111/pde.15461 [17] 张雨桐,宋杨,刘凤凤,等. 新型冠状病毒感染“乙类乙管”后我国手足口病流行特征与趋势分析[J]. 热带病与寄生虫学,2023,21(4):186-190+227. doi: 10.3969/j.issn.1672-2302.2023.04.002 [18] Li Y Z,Ruan Y,Zhai X J,et al. Frontiers and hotspots in hand,foot,and mouth disease research during 2006 to 2023: A bibliometric and visual analysis[J]. Medicine,2024,103(24):e38550. [19] Rajamoorthy Y,Taib N M,Harapan H,et al. Application of the double bounded dichotomous choice model to the estimation of parent's willingness to pay for the hand foot mouth disease vaccination: A survey in Selangor,Malaysia[J]. PloS One,2023,18(6):e0286924. doi: 10.1371/journal.pone.0286924 [20] Duan X X,Zhang L Z,Ding L,et al. Effectiveness of enterovirus A71 vaccine against pediatric HFMD and disease profile of post-vaccination infection[J]. Vaccine,2024,42(9):2317-2325. doi: 10.1016/j.vaccine.2024.02.026 [21] Zhu P Y,Ji W Q,Li D,et al. Current status of hand-foot-and-mouth disease[J]. Journal of Biomedical Science,2023,30(1):15. doi: 10.1186/s12929-023-00908-4 -





 下载:
下载: