Progress and Challenges in the Study of Bacteriophage Therapy for Carbapenem-Resistant Klebsiella Pneumoniae Infection
-
摘要: 碳青霉烯类耐药肺炎克雷伯菌(CRKP)作为超级耐药病原体,已成为全球公共卫生的重要威胁,尤其在ICU和免疫低下患者中检出率显著升高。传统抗生素疗效递减,CRKP耐药谱持续扩大,临床治疗面临巨大挑战。噬菌体作为特异性感染细菌的病毒,因其裂解能力强、生物膜穿透性好、副作用低、能协同抗生素发挥治疗作用等特点,成为替代或补充抗生素治疗的新策略。系统综述CRKP的流行病学和耐药机制、噬菌体治疗的原理与机制、研究进展(含体外实验、动物模型和临床应用)、联合疗法及其优势与挑战,并结合最新研究提出未来发展方向,包括基因工程噬菌体的构建、合成生物学设计的“智能噬菌体”、以及临床监管体系的建立。噬菌体疗法为抗击CRKP提供了潜在突破口,但其标准化、广谱性与临床转化路径仍需深入探索与规范。
-
关键词:
- 碳青霉烯类耐药肺炎克雷伯菌 /
- 抗生素 /
- 噬菌体 /
- 噬菌体疗法
Abstract: Carbapenem-resistant Klebsiella pneumoniae (CRKP) , as a superbug pathogen, has become a critical global public health threat, with significantly increased detection rates especially in ICU and immunocompromised patients. Traditional antibiotics are progressively losing efficacy, and CRKP's antibiotic resistance spectrum continues to expand, presenting enormous clinical treatment challenges. Bacteriophages, as viruses specifically infecting bacteria, have emerged as a new therapeutic strategy for alternative or complementary antibiotic treatment due to their strong lytic capabilities, excellent biofilm penetration, low side effects, and ability to synergize with antibiotics. This article systematically reviews the epidemiology and resistance mechanisms of CRKP, principles and mechanisms of bacteriophage therapy, research progress (including in vitro experiments, animal models, and clinical applications), combined therapies, and their advantages and challenges. By incorporating the latest research, the article also proposes future development directions, including constructing genetically engineered bacteriophages, designing "intelligent bacteriophages" through synthetic biology, and establishing clinical regulatory systems. Bacteriophage therapy offers a potential breakthrough in combating CRKP, but its standardization, broad-spectrum applicability, and clinical translation pathways still require in-depth exploration and standardization. -
表 1 噬菌体VS抗生素机制对比表
Table 1. Comparison of bacteriophage vs. antibiotic mechanisms
机制维度 噬菌体机制 抗生素机制 临床意义 识别机制 RBP识别细菌K抗原、LPS等 广谱靶向细胞壁或蛋白合成 精准治疗CRKP定植感染,避免广谱抗生素导致的菌群失调 杀菌方式 裂解性感染,释放裂解酶引发宿主破裂 阻断关键代谢路径导致细菌死亡 噬菌体裂解可能释放内毒素(需监测炎症),抗生素更适急性脓毒症 生物膜作用 可分泌多糖酶,穿透EPS 多数抗生素无法穿透成熟生物膜 噬菌体对导管相关感染/骨髓炎更具优势 耐药机制规避 可更换噬菌体,适应突变,个性化组合 易产生酶解、外排、
靶点突变等耐药个性化疗法解决MDR-CRKP,但需快速菌株匹配平台 毒副作用控制 几乎无毒,宿主特异性高 可引发肝肾毒性、二重感染等 噬菌体适合免疫低下患者,但需警惕IgG中和 与免疫系统互作 可被抗体中和;部分递送方式可延缓清除 通常不会直接被免疫系统清除 静脉噬菌体需纳米载体递送,黏膜给药延长驻留 协同策略 与抗生素、内溶素、益生菌联合使用增强效果 与其他抗生素联合
可增强或诱导耐药噬菌体+抗生素用于对抗生物膜 表 2 噬菌体治疗CRKP感染的研究进展汇总(体外实验、动物模型与临床应用)
Table 2. Research advances in phage therapy for CRKP infection (in vitro experiments,animal models and clinical applications)
研究类型 研究模型/菌株 噬菌体/疗法 剂量/MOI 给药方式 主要结果 局限性 参考文献 体外实验 K1血清型CRKP菌株 噬菌体vB_KpnP_ZK1 + 解聚酶Depo16 10 MOI 体外共培养 去除K1型CRKP荚膜多糖,
小鼠存活率提高80%仅对K1血清型有效 [32] 多重耐药肺炎克雷伯菌 噬菌体ZCKP1 50 PFU/CFU 静态生物膜模型 细菌载量↓≥2 log10 CFU/mL,
生物膜生物量↓>50%高剂量可能引发免疫反应 [33] 人类IBD肠道类器官 五噬菌体鸡尾酒 107 PFU/mL 共培养 选择性清除Kp,保留其他共生菌,
同时避免了耐药性的产生未测试临床患者 [34] 动物模型 高产酒精CRKP诱导的小鼠脂肪肝 噬菌体phiW14 108 PFU/只 腹腔注射 肝脏病理损伤减轻,血清ALT/AST↓,
白细胞介素(IL)-10的表达上调,
肠道菌群未失衡未测试长期毒性 [35] 产气克雷伯菌肺炎小鼠模型 噬菌体pk4-26 + 解聚酶 107 PFU/只 鼻腔滴注 肺组织细菌载量↓90%,炎症因子TNF-α↓ 未验证其他血清型 [36] IBD小鼠模型(Kp定植) 五噬菌体鸡尾酒 109 PFU/只 口服灌胃 肠道Kp载量↓90%,结肠炎症评分↓50% 未明确包含CRKP菌株 [34] 临床应用 CRKP假体膝关节感染(KPC-2阳性) 噬菌体KpJH46 + 米诺环素 107 PFU/mL 局部灌注噬菌体 + 口服抗生素 症状完全缓解,生物膜清除率>60%
(体外),联用后杀菌效率↑3倍;该案例
推动FDA批准扩大噬菌体临床试验单病例,未验证其他菌株 [37] 肾移植受者(ESBL-KP复发性UTI) 三噬菌体鸡尾酒
(Metamorpho/Mineola/
pKp20)5×109 PFU/次,每日2次 静脉注射(4周) 治疗后6个月无ESBL-KP复发,
1年后仅出现敏感株感染;
血清中和反应轻微(半衰期0.4 h)单病例,未评估肠道定植清除 [38] 医院RICU环境表面(ST11 KL47/KL64型CRKP) 噬菌体鸡尾酒(1株抗KL47 + 2株抗KL64) 108 PFU/mL(雾化后
107 PFU/mL)超声雾化(500 mL/房间) 24 h内CRKP载量↓60%(ddPCR检测),
72 h内维持低水平;微生物群落
多样性未受干扰48h后出现轻微反弹,需定期重复应用 [39] 联合疗法 泛耐药CRKP体外模型 预适应噬菌体 + 美罗培南 + 粘菌素 1 MOI + 抗生素 动态培养系统 协同杀菌效率↑3倍,延缓耐药性出现 未测试人体药代动力学 [40] CRKP生物膜感染(体外) 噬菌体KpV74 + 解聚酶Dep_kpv74 20 MOI 生物膜破坏实验 解聚酶对多血清型有效,
噬菌体仅对支持繁殖的菌株有效解聚酶体内效果未验证 [41] ST11 K64 CRKP生物膜 解聚酶Dep37 + 卡那霉素 Dep37: 10 μg/mL; 卡那霉素: 4~8 μg/mL 体外生物膜破坏实验 联合治疗显著降低细菌载量,
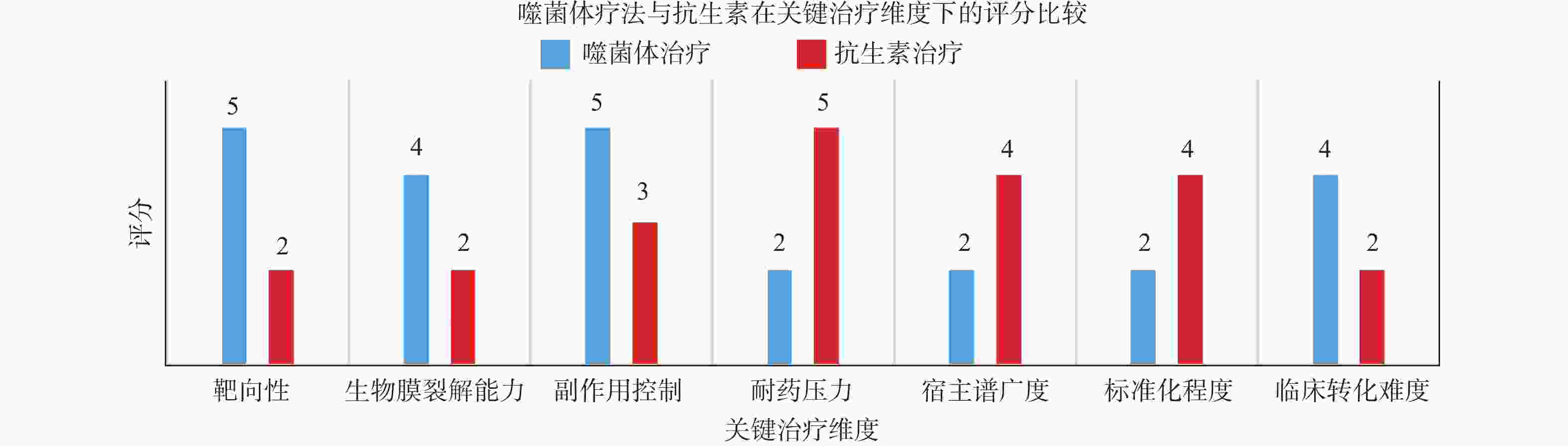
效果优于单一治疗解聚酶体内效果未验证,未测试其他抗生素组合 [42] 表 3 噬菌体疗法与抗生素在关键治疗维度下的评分对比及评分依据说明
Table 3. Comparison of phage therapy and antibiotic scores under key therapeutic dimensions and description of the basis for the scores
维度 噬菌体评分
(1~5)抗生素评分
(1~5)评分依据说明 靶向性 5 2 噬菌体通常针对特定菌株(高度专一),不会破坏正常菌群,抗生素广谱杀菌,影响肠道微生态,相较抗生素的广谱效应更具选择性 生物膜裂解能力 4 2 多种噬菌体可表达多糖酶或穿孔酶,对细菌生物膜具有强穿透力,而抗生素难穿透生物膜易失效 副作用控制 5 3 噬菌体为天然病毒,不易引起全身毒副反应,抗生素常引发肝肾毒性、过敏反应等副作用 耐药压力 2 5 噬菌体对菌群世家选择压力较小,且可变换组合;抗生素长期使用诱导多种耐药机制产生 宿主普广度 2 4 天然噬菌体宿主普较窄,需筛选;抗生素具有较广覆盖面,但可能影响有益菌群 标准化程度 2 4 噬菌体缺乏统一质量控制标准,常为个体化制备;抗生素已有完善的审批流程、质量标准和生产体系 临床转化难度 4 2 噬菌体疗法在个别国家已有尝试性应用经验,但未规模化;抗生素疗法监管体系完善,已建立药典标准、指南、伦理规范 -
[1] Chen Q,Wang M,Han M,et al. Molecular basis of Klebsiella pneumoniae colonization in host[J]. Microbial Pathogenesis,2023,177:106026. doi: 10.1016/j.micpath.2023.106026 [2] 李海源. 肺炎克雷伯菌乳房炎的致病机理及其耐药性分析[J]. 中国乳业,2023,(11):39-44. doi: 10.12377/1671-4393.23.11.08 [3] Han Y L,Wen X H,Zhao W,et al. Epidemiological characteristics and molecular evolution mechanisms of carbapenem-resistant hypervirulent Klebsiella pneumoniae[J]. Frontiers in Microbiology,2022,13:1003783. doi: 10.3389/fmicb.2022.1003783 [4] Śliwka P,Ochocka M,Skaradzińska A. Applications of bacteriophages against intracellular bacteria[J]. Critical Reviews in Microbiology,2021,48(2):222-239. [5] Dunne M,Rupf B,Tala M,et al. Reprogramming bacteriophage host range through structure-guided design of chimeric receptor binding proteins [J]. Cell Reports,2019,29(5): 1336-1350. e4. [6] Zurabov F,Zhilenkov E. Characterization of four virulent Klebsiella pneumoniae bacteriophages,and evaluation of their potential use in complex phage preparation[J]. Virology Journal,2021,18(1):9. doi: 10.1186/s12985-020-01485-w [7] 周彤,周秀娟,龙坤兰,等. 肺炎克雷伯菌生物膜治疗的研究进展[J]. 中国抗生素杂志,2023,48:636-642. doi: 10.3969/j.issn.1001-8689.2023.06.005 [8] Olsen I. Biofilm-specific antibiotic tolerance and resistance[J]. European Journal of Clinical Microbiology & Infectious Diseases,2015,34(5):877-886. [9] 郭燕,胡付品,朱德妹,等. 2023年CHINET中国细菌耐药监测[J]. 中国感染与化疗杂志,2024,24(6):627-637. [10] Hu F,Pan Y,Li H,et al. Carbapenem-resistant Klebsiella pneumoniae capsular types,antibiotic resistance and virulence factors in China: A longitudinal,multi-centre study[J]. Nat Microbiol,2024,9(3):814-829. doi: 10.1038/s41564-024-01612-1 [11] Yang X,Sun Q,Li J,et al. Molecular epidemiology of carbapenem-resistant hypervirulent Klebsiella pneumoniae in China[J]. Emerging Microbes & Infections,2022,11(1):841-849. [12] Zhang R,Liu L,Zhou H,et al. Nationwide surveillance of Clinical Carbapenem-resistant Enterobacteriaceae (CRE) strains in China[J]. EBioMedicine,2017,19:98-106. doi: 10.1016/j.ebiom.2017.04.032 [13] Conlan S,Thomas P J,Deming C,et al. Single-molecule sequencing to track plasmid diversity of hospital-associated carbapenemase-producing Enterobacteriaceae[J]. Science Translational Medicine,2014,6(254):254ra126. [14] Pu D,Zhao J,Chang K,et al. "Superbugs" with hypervirulence and carbapenem resistance in Klebsiella pneumoniae: the rise of such emerging nosocomial pathogens in China[J]. Science Bulletin,2023,68(21):2658-2670. doi: 10.1016/j.scib.2023.09.040 [15] Strathdee S A,Hatfull G F,Mutalik V K,et al. Phage therapy: From biological mechanisms to future directions[J]. Cell,2023,186(1):17-31. doi: 10.1016/j.cell.2022.11.017 [16] Toporek A,Lechtzin N. Viruses to the rescue-Use of bacteriophage to treat resistant pulmonary infections[J]. Cell,2022,185(11):1807-1808. doi: 10.1016/j.cell.2022.04.037 [17] Hu B,Margolin W,Molineux I J,et al. The bacteriophage t7 virion undergoes extensive structural remodeling during infection[J]. Science,2013,339(6119):576-579. doi: 10.1126/science.1231887 [18] Tzipilevich E,Habusha M,Ben-Yehuda S. Acquisition of phage sensitivity by bacteria through exchange of phage receptors [J]. Cell,2017,168(1-2): 186-199. e12. [19] Federici S,Nobs S P,Elinav E. Phages and their potential to modulate the microbiome and immunity[J]. Cellular & Molecular Immunology,2021,18(4):889-904. [20] Karygianni L,Ren Z,Koo H,et al. Biofilm matrixome: Extracellular components in structured microbial communities[J]. Trends in Microbiology,2020,28(8):668-681. doi: 10.1016/j.tim.2020.03.016 [21] Gordon M,Ramirez P. Efficacy and experience of bacteriophages in biofilm-related infections[J]. Antibiotics (Basel,Switzerland),2024,13(2):125. doi: 10.3390/antibiotics13020125 [22] Tkhilaishvili T,Winkler T,Müller M,et al. Bacteriophages as adjuvant to antibiotics for the treatment of periprosthetic joint infection caused by multidrug-resistant pseudomonas aeruginosa[J]. Antimicrobial Agents and Chemotherapy,2019,64(1):e00924-19. [23] Ferry T,Leboucher G,Fevre C,et al. Salvage Debridement,Antibiotics and Implant Retention ("DAIR") with local injection of a selected cocktail of bacteriophages: Is it an option for an elderly patient with relapsing staphylococcus aureus prosthetic-joint infection?[J]. Open Forum Infectious Diseases,2018,5(11):ofy269. doi: 10.1093/ofid/ofy269 [24] Fang Q,Yin X,He Y,et al. Safety and efficacy of phage application in bacterial decolonisation: A systematic review[J]. The Lancet Microbe,2024,5(5):e489-e499. doi: 10.1016/S2666-5247(24)00002-8 [25] Guo Z,Liu M,Zhang D. Potential of phage depolymerase for the treatment of bacterial biofilms[J]. Virulence,2023,14(1):2273567. doi: 10.1080/21505594.2023.2273567 [26] Hossain A A,Pigli Y Z,Baca C F,et al. DNA glycosylases provide antiviral defence in prokaryotes[J]. Nature,2024,629(8011):410-416. doi: 10.1038/s41586-024-07329-9 [27] Berngruber T W,Lion S,Gandon S. Evolution of suicide as a defence strategy against pathogens in a spatially structured environment[J]. Ecology Letters,2013,16(4):446-453. doi: 10.1111/ele.12064 [28] Hör J,Wolf S G,Sorek R. Bacteria conjugate ubiquitin-like proteins to interfere with phage assembly[J]. Nature,2024,631(8022):850-856. doi: 10.1038/s41586-024-07616-5 [29] Reina J,Reina N. Phage therapy,an alternative to antibiotic therapy?[J]. Revista Espanolade Quimioterapia,2018,31(2):101-104. [30] Gordillo Altamirano F L,Barr J J. Phage therapy in the postantibiotic era[J]. Clinical Microbiology Reviews,2019,32(2):e00066-18. [31] Kebriaei R,Lev K L,Shah R M,et al. Eradication of biofilm-mediated methicillin-resistant staphylococcus aureus infections in vitro: Bacteriophage-antibiotic combination[J]. Microbiology Spectrum,2022,10(2):e0041122. doi: 10.1128/spectrum.00411-22 [32] Zhao R,Jiang S,Ren S,et al. A novel phage putative depolymerase,Depo16,has specific activity against K1 capsular-type Klebsiella pneumoniae[J]. Applied and Environmental Microbiology,2024,90(4):e0119723. doi: 10.1128/aem.01197-23 [33] Taha O A,Connerton P L,Connerton I F,et al. Bacteriophage ZCKP1: A potential treatment for Klebsiella pneumoniae isolated from diabetic foot patients[J]. Frontiers in Microbiology,2018,9:2127. doi: 10.3389/fmicb.2018.02127 [34] Federici S,Kredo-Russo S,Valdés-Mas R,et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation [J]. Cell,2022,185(16): 2879-2898. e24. [35] Gan L,Feng Y,Du B,et al. Bacteriophage targeting microbiota alleviates non-alcoholic fatty liver disease induced by high alcohol-producing Klebsiella pneumoniae[J]. Nat Commun,2023,14(1):3215. doi: 10.1038/s41467-023-39028-w [36] Cui X,Du B,Feng J,et al. A novel phage carrying capsule depolymerase effectively relieves pneumonia caused by multidrug-resistant Klebsiella aerogenes[J]. Journal of Biomedical Science,2023,30(1):75. doi: 10.1186/s12929-023-00946-y [37] Cano E J,Caflisch K M,Bollyky P L,et al. Phage therapy for limb-threatening prosthetic knee Klebsiella pneumoniae infection: Case report and in vitro characterization of anti-biofilm activity[J]. Clinical Infectious Diseases,2021,73(1):e144-e151. doi: 10.1093/cid/ciaa705 [38] Le T,Nang S C,Zhao J,et al. Therapeutic potential of intravenous phage as stand alone therapy for recurrent drug-resistant urinary tract infections[J]. Antimicrobial Agents and Chemotherapy,2023,67(4):e0003723. doi: 10.1128/aac.00037-23 [39] Shi Y,Zhang W,Li L,et al. Evaluation of phage-based decontamination in respiratory intensive care unit environments using ddPCR and 16S rRNA targeted sequencing techniques[J]. Frontiers in Cellular and Infection Microbiology,2024,14:1442062. doi: 10.3389/fcimb.2024.1442062 [40] Eskenazi A,Lood C,Wubbolts J,et al. Combination of pre-adapted bacteriophage therapy and antibiotics for treatment of fracture-related infection due to pandrug-resistant Klebsiella pneumoniae[J]. Nat Commun,2022,13(1):302. doi: 10.1038/s41467-021-27656-z [41] Volozhantsev N V,Borzilov A I,Shpirt A M,et al. Comparison of the therapeutic potential of bacteriophage KpV74 and phage-derived depolymerase (β-glucosidase) against Klebsiella pneumoniae capsular type K2[J]. Virus Research,2022,322:198951. doi: 10.1016/j.virusres.2022.198951 [42] Yang P,Shan B,Hu X,et al. Identification of a novel phage depolymerase against ST11 K64 carbapenem-resistant Klebsiella pneumoniae and its therapeutic potential[J]. Journal of Bacteriology,2025,207(4):e0038724. doi: 10.1128/jb.00387-24 [43] 姜昕宇,吴楠楠,卢曙光,等. 噬菌体临床应用监管法规的现状及展望[J]. 中国抗生素杂志,2024,49(1):26-34. doi: 10.3969/j.issn.1001-8689.2024.01.003 [44] Taati Moghadam M,Amirmozafari N,Shariati A,et al. How phages overcome the challenges of drug resistant bacteria in clinical infections[J]. Infection and Drug Resistance,2020,13:45-61. doi: 10.2147/IDR.S234353 [45] Chakraborty S,Rohit A,Prasanthi S J,et al. A New casjensviridae bacteriophage isolated from hospital sewage for inactivation of biofilms of carbapenem resistant Klebsiella pneumoniae clinical isolates[J]. Pharmaceutics,2024,16(7):904. doi: 10.3390/pharmaceutics16070904 [46] Hibstu Z,Belew H,Akelew Y,et al. Phage therapy: A different approach to fight bacterial infections [J]. Biologics : Targets & Therapy,2022,16: 173-186. [47] Liu D,Van Belleghem J D,de Vries C R,et al. The safety and toxicity of phage therapy: A review of animal and clinical studies[J]. Viruses,2021,13(7):1268. doi: 10.3390/v13071268 [48] Dedrick R M,Guerrero-Bustamante C A,Garlena R A,et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant mycobacterium abscessus[J]. Nature Medicine,2019,25(5):730-733. doi: 10.1038/s41591-019-0437-z [49] Kortright K E,Chan B K,Koff J L,et al. Phage therapy: A renewed approach to combat antibiotic-resistant bacteria[J]. Cell Host & Microbe,2019,25(2):219-232. [50] Ando H,Lemire S,Pires D P,et al. Engineering modular viral scaffolds for targeted bacterial population editing[J]. Cell Systems,2015,1(3):187-196. doi: 10.1016/j.cels.2015.08.013 [51] Kilcher S,Studer P,Muessner C,et al. Cross-genus rebooting of custom-made,synthetic bacteriophage genomes in L-form bacteria[J]. Proceedings of the National Academy of Sciences of the United States of America,2018,115(3):567-572. [52] Schooley R T,Biswas B,Gill J J,et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant acinetobacter baumannii infection[J]. Antimicrobial Agents and Chemotherapy,2017,61(10):e00954-17. [53] Letkiewicz S,Łusiak-Szelachowska M,Międzybrodzki R,et al. Low immunogenicity of intravesical phage therapy for urogenitary tract infections[J]. Antibiotics (Basel,Switzerland),2021,10(6):627. doi: 10.3390/antibiotics10060627 -





 下载:
下载: