Activation of γ-Aminobutyric Acid Type A Receptor Attenuates Oxygen-glucose Deprivation/Reoxygenation-induced Astrocyte Injury
-
摘要:
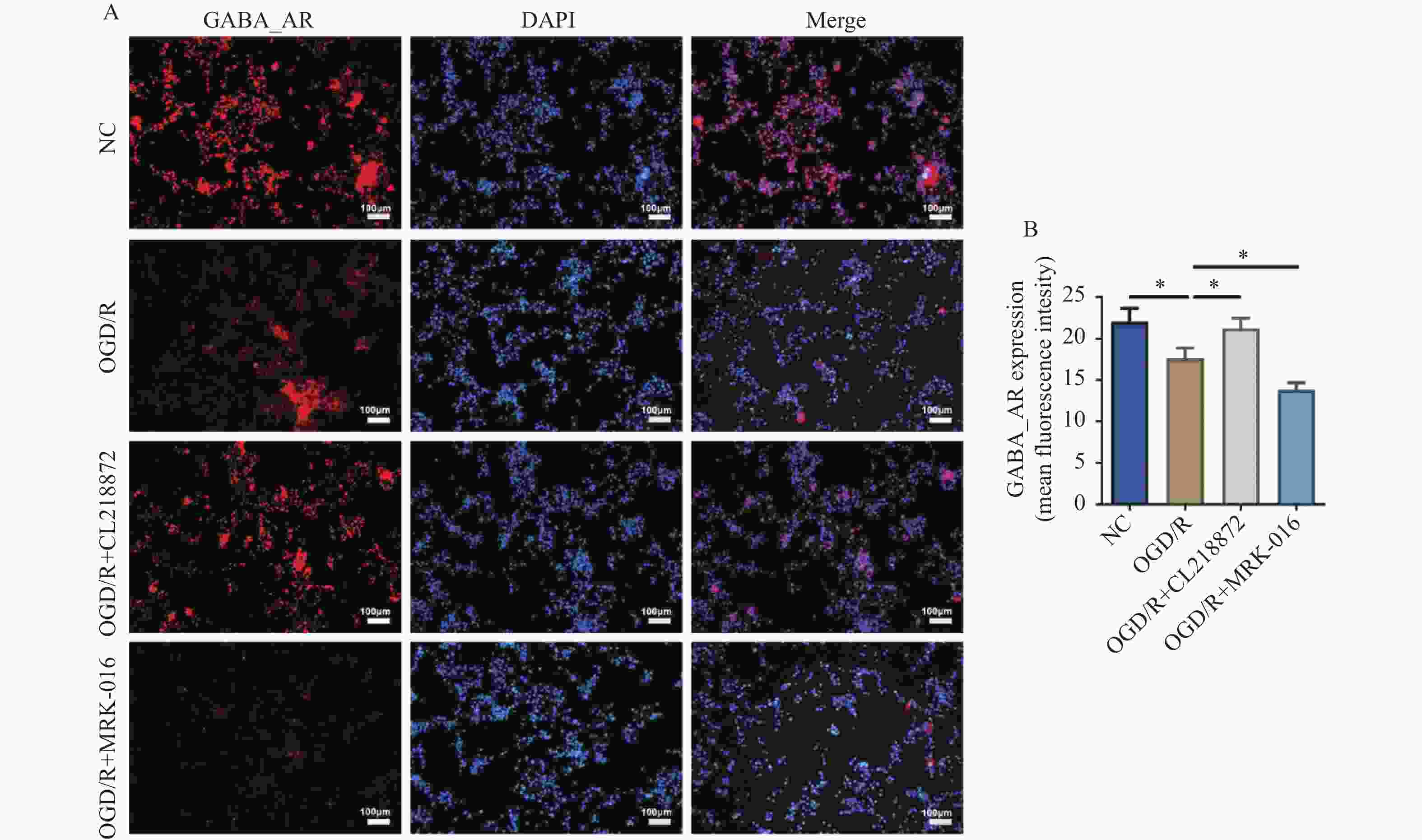
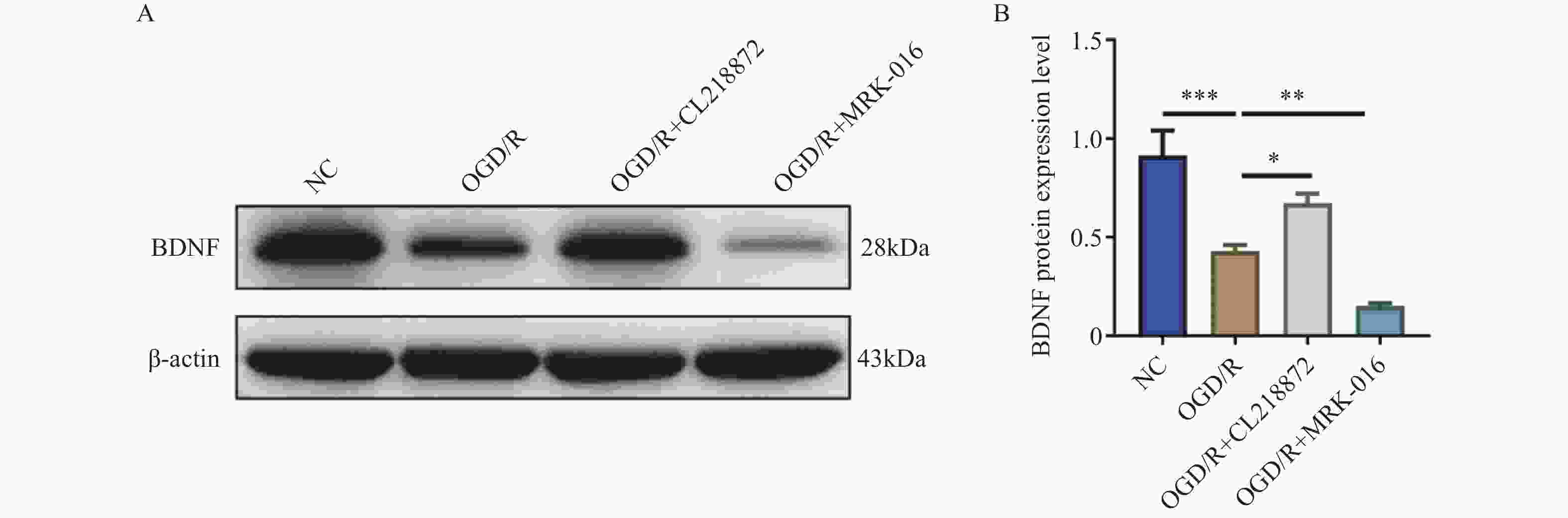
目的 探讨γ-氨基丁酸A型受体(γ-aminobutyric acid type A receptor,GABA_AR)对氧糖剥夺/复氧(oxygen-glucose deprivation/reoxygenation,OGD/R)诱导的星形胶质细胞损伤的保护作用及潜在机制。 方法 采用C8-D1A星形胶质细胞建立OGD/R损伤模型,随机分为正常对照组(NC)、OGD/R模型组、GABA_AR激动剂CL218872干预组(OGD/R+CL218872)及抑制剂MRK-016干预组(OGD/R+MRK-016)。通过EdU染色法检测细胞增殖;流式细胞术分析细胞凋亡;免疫荧光染色检测GABA_AR及胶质纤维酸性蛋白(glial fibrillary acidic protein,GFAP)的表达;Western blot法检测脑源性神经营养因子(brain-derived neurotrophic factor,BDNF)的表达。 结果 与OGD/R组比较,OGD/R+CL218872组细胞增殖率显著增强(P < 0.05),凋亡率明显降低(P < 0.01),GABA_AR与GFAP表达均上调(P < 0.05),BDNF蛋白表达水平显著升高(P < 0.05),炎症因子IL-6(P < 0.01)与TNF-α(P < 0.05)水平显著减少。而OGD/R+MRK-016组各指标均呈相反变化。 结论 GABA_AR激活可通过促进星形胶质细胞增殖、抑制其凋亡与炎症反应,并上调BDNF与GFAP的表达,从而减轻OGD/R诱导的细胞损伤。 -
关键词:
- γ-氨基丁酸A型受体 /
- 星形胶质细胞 /
- 缺糖缺氧/复糖复氧 /
- 突触重塑 /
- 细胞凋亡
Abstract:Objective To investigate the protective role and potential mechanisms of γ-aminobutyric acid type A receptor (GABA_AR) against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced astrocyte injury. Methods C8-D1A astrocytes were used to establish an OGD/R injury model, which was randomly divided into four groups: the normal control group (NC), the OGD/R model group, the GABA_AR agonist CL218872 intervention group (OGD/R+CL218872), and the inhibitor MRK-016 intervention group (OGD/R+MRK-016). Cell proliferation was assessed by EdU staining, apoptosis was analyzed by flow cytometry, GABA_AR and glial fibrillary acidic protein (GFAP) expression were examined by immunofluorescence staining, and brain-derived neurotrophic factor (BDNF) expression was determined by Western blotting. Results Compared with the OGD/R group, the OGD/R+CL218872 group showed significantly increased cell proliferation rate (P < 0.05) , markedly decreased apoptosis rate(P < 0.01), upregulated expression of both GABA_AR and GFAP(P < 0.05) significantly elevated BDNF protein expression level(P < 0.05), and significantly reduced levels of inflammatory factors IL-6 (P < 0.01) and TNF-α (P < 0.05). The OGD/R+MRK-016 group showed opposite changes in all parameters. Conclusion GABA_AR activation can attenuate OGD/R-induced astrocyte injury by promoting astrocyte proliferation, inhibiting apoptosis and inflammatory response, and upregulating the expression of BDNF and GFAP. -
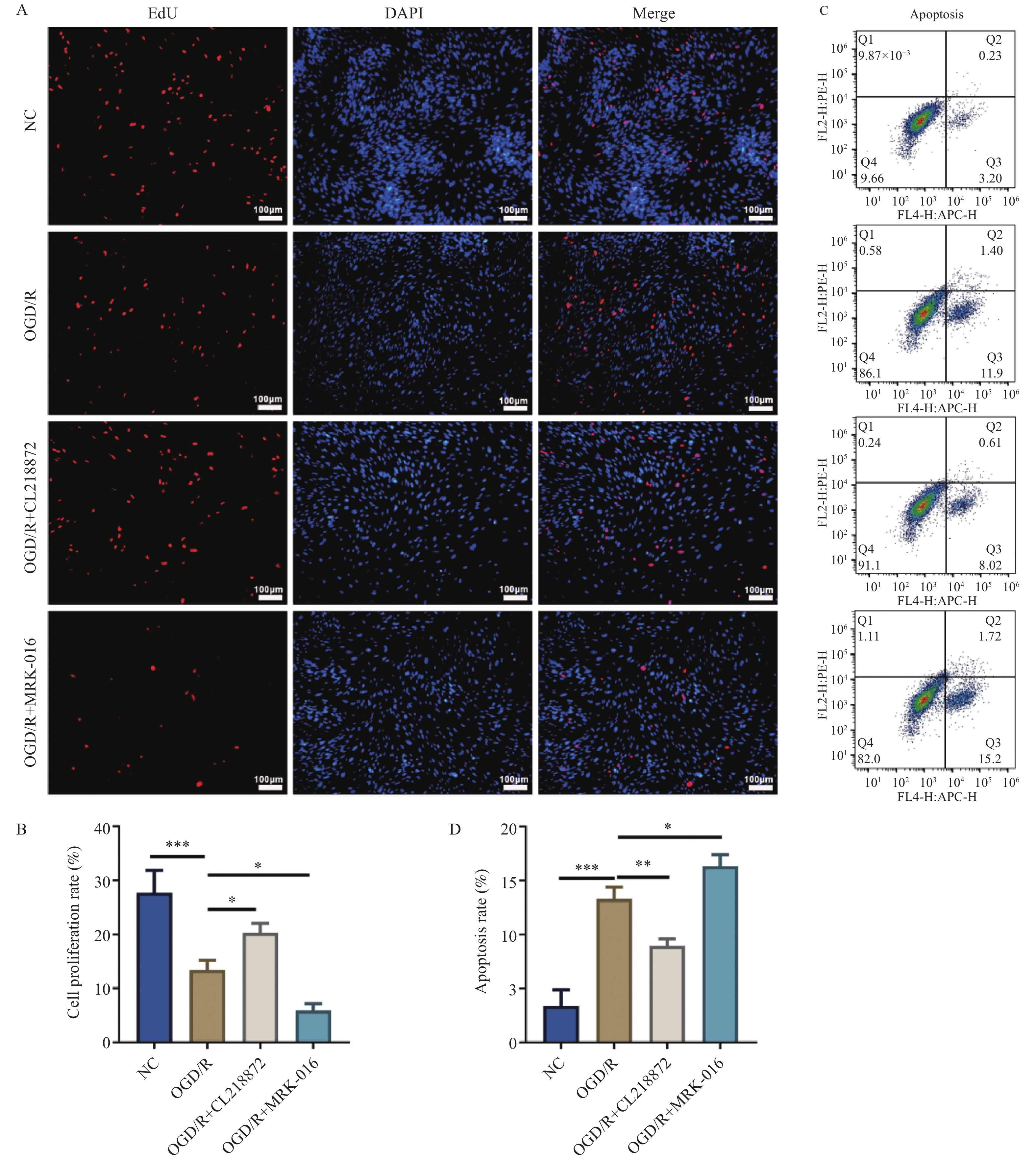
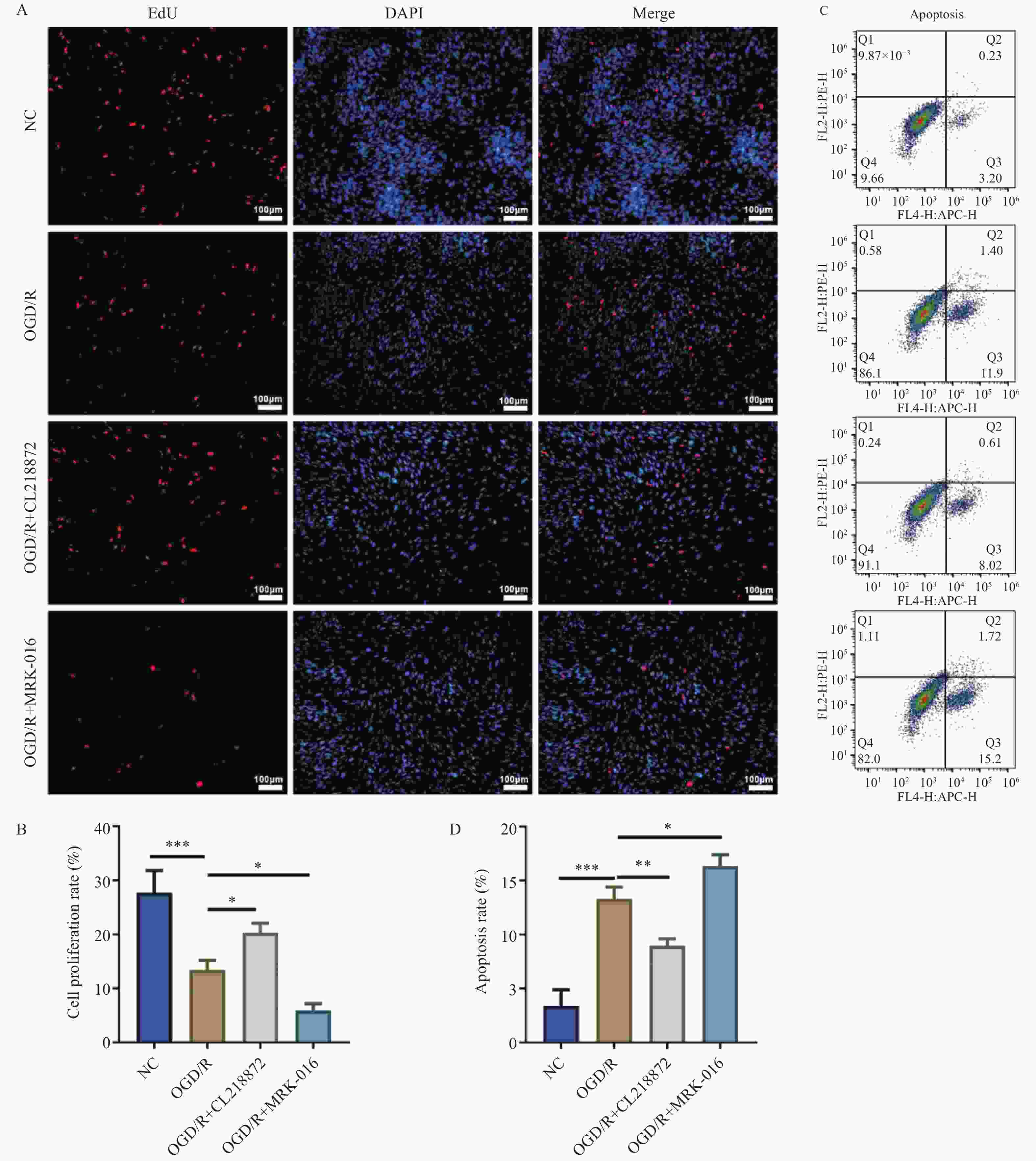
图 1 EdU染色与流式细胞术分析AS的增殖与凋亡水平[($ \bar x \pm s $),n = 3]
A:EdU染色的代表性图片(100×,比例尺:100 μm);B:细胞增殖率统计结果;C:流式细胞术检测细胞凋亡的散点图;D:细胞凋亡率统计结果;蓝色荧光为细胞核,红色荧光为增殖细胞;***P < 0.001;**P < 0.01;*P < 0.05。
Figure 1. Analysis of proliferation and apoptosis levels in AS by Edu staining and flow cytometry [($ \bar x \pm s $),n = 3]
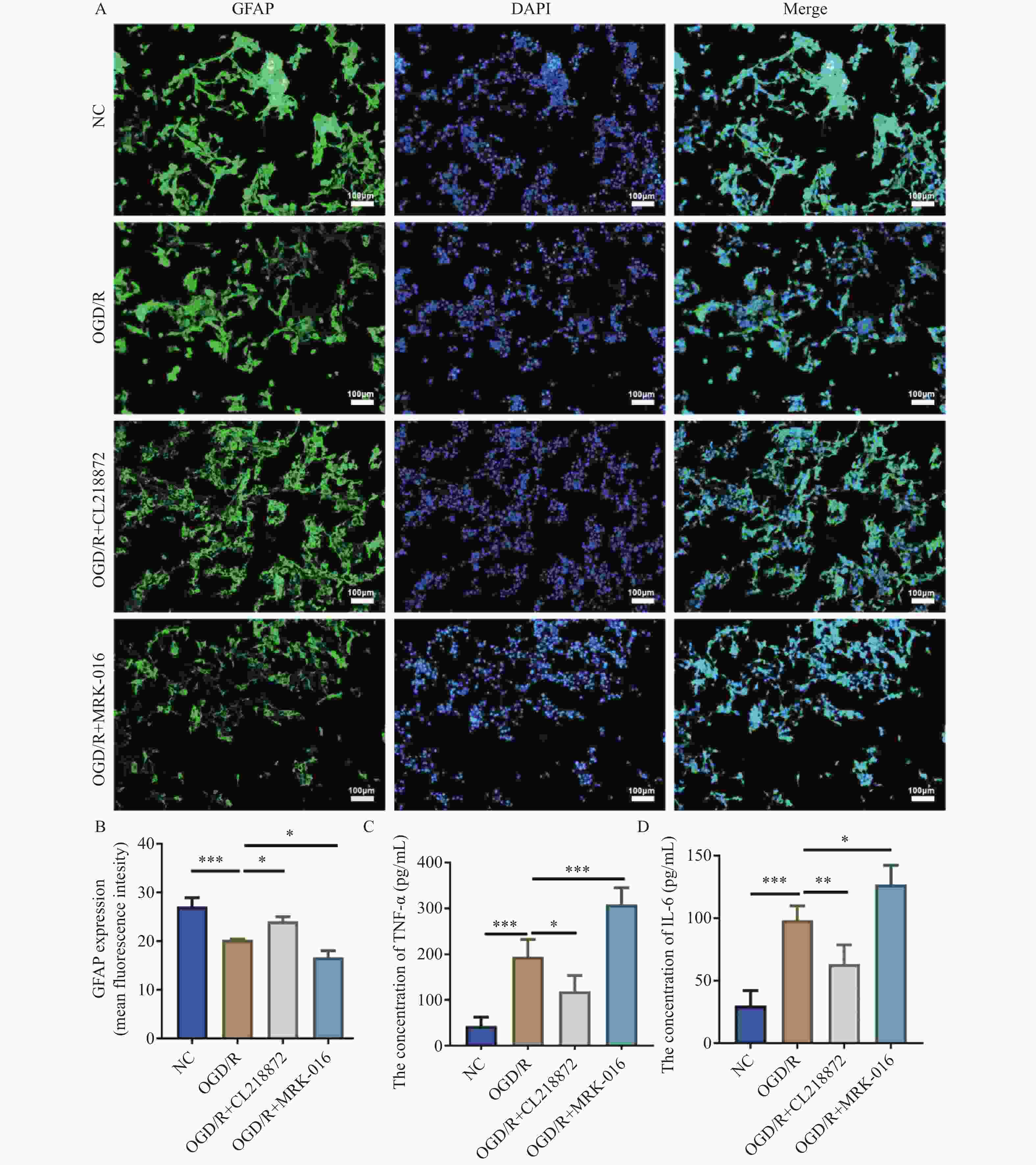
图 3 GABA_AR调节对AS中GFAP表达及炎症因子释放的影响[($ \bar x \pm s $),n = 5]
A:GFAP免疫荧光染色图像(100×,比例尺:100 μm),蓝色为细胞核,绿色为GFAP;B:GFAP表达的统计分析;C:AS中TNF-α水平;D:AS中IL-6水平;***P < 0.001;**P < 0.01;*P < 0.05。
Figure 3. Effects of GABA_AR modulation on GFAP expression and inflammatory factor release in AS [($ \bar x \pm s $),n = 5]
-
[1] Zhang M, Liu Q, Meng H, et al. Ischemia-reperfusion injury: Molecular mechanisms and therapeutic targets[J]. Signal Transduct Target Ther, 2024, 9(1): 12. doi: 10.1038/s41392-023-01688-x [2] Wu L, Xiong X, Wu X, et al. Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury[J]. Front Mol Neurosci, 2020, 13: 28. doi: 10.3389/fnmol.2020.00028 [3] Zhang Q, Jia M, Wang Y, et al. Cell death mechanisms in cerebral ischemia-reperfusion injury[J]. Neurochem Res, 2022, 47(12): 3525-3542. doi: 10.1007/s11064-022-03697-8 [4] Burda J E, Bernstein A M, Sofroniew M V. Astrocyte roles in traumatic brain injury[J]. Exp Neurol, 2016, 275 Pt 3(3): 305-315. [5] George J, Lu Y, Tsuchishima M, et al. Cellular and molecular mechanisms of hepatic ischemia-reperfusion injury: The role of oxidative stress and therapeutic approaches[J]. Redox Biol, 2024, 75: 103258. doi: 10.1016/j.redox.2024.103258 [6] Wang H, Qiao Y, Lu H, et al. Astrocyte autophagy-neuroinflammation axis in ischemic stroke: From molecular mechanisms to translational medicine[J]. Neuroscience, 2025, 589: 128-139. doi: 10.1016/j.neuroscience.2025.10.036 [7] Liu H, Wu X, Luo J, et al. Pterostilbene attenuates astrocytic inflammation and neuronal oxidative injury after ischemia-reperfusion by inhibiting NF-κB phosphorylation[J]. Front Immunol, 2019, 10: 2408. doi: 10.3389/fimmu.2019.02408 [8] Wicha P, Tocharus J, Janyou A, et al. Hexahydrocurcumin alleviated blood-brain barrier dysfunction in cerebral ischemia/reperfusion rats[J]. Pharmacol Rep, 2020, 72(3): 659-671. doi: 10.1007/s43440-019-00050-9 [9] Andersen J V, Schousboe A, Wellendorph P. Astrocytes regulate inhibitory neurotransmission through GABA uptake, metabolism, and recycling[J]. Essays Biochem, 2023, 67(1): 77-91. doi: 10.1042/EBC20220208 [10] Ouyang C, Guo L, Lu Q, et al. Enhanced activity of GABA receptors inhibits glutamate release induced by focal cerebral ischemia in rat striatum[J]. Neurosci Lett, 2007, 420(2): 174-178. doi: 10.1016/j.neulet.2007.05.004 [11] Michalettos G, Ruscher K. Crosstalk between GABAergic neurotransmission and inflammatory cascades in the post-ischemic brain: Relevance for stroke recovery[J]. Front Cell Neurosci, 2022, 16: 807911. doi: 10.3389/fncel.2022.807911 [12] Zhang X, Yan H, Yuan Y, et al. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance[J]. Autophagy, 2013, 9(9): 1321-1333. doi: 10.4161/auto.25132 [13] Liu J, Feng X, Wang Y, et al. Astrocytes: GABAceptive and GABAergic cells in the brain[J]. Front Cell Neurosci, 2022, 16: 892497. doi: 10.3389/fncel.2022.892497 [14] Li L, Zhou J, Sun H, et al. A computational model to investigate GABA-activated astrocyte modulation of neuronal excitation[J]. Comput Math Meth Med, 2020, 2020(1): 8750167. [15] Zhi S M, Fang G X, Xie X M, et al. Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling[J]. Eur Rev Med Pharmacol Sci, 2020, 24(3): 1524-1536. [16] Deng Y, Duan R, Ding W, et al. Astrocyte-derived exosomal nicotinamide phosphoribosyltransferase (Nampt) ameliorates ischemic stroke injury by targeting AMPK/mTOR signaling to induce autophagy[J]. Cell Death Dis, 2022, 13(12): 1057. doi: 10.1038/s41419-022-05454-9 [17] Wang Y J, Seibert H, Ahn L Y, et al. Pharmacological chaperones restore proteostasis of epilepsy-associated GABA(A) receptor variants[J]. Pharmacol Res, 2024, 208: 107356. doi: 10.1016/j.phrs.2024.107356 [18] Lecker I, Yin Y, Wang D S, et al. Potentiation of GABAA receptor activity by volatile anaesthetics is reduced by α5GABAA receptor-preferring inverse agonists[J]. Br J Anaesth, 2013, 110(Suppl 1): i73-i81. [19] Li Y, Yang H, Liu L, et al. From neurotoxicity to neuroprotection: Rethinking GABA(a)R-targeting anesthetics[J]. Cell Biol Toxicol, 2025, 41(1): 104. doi: 10.1007/s10565-025-10057-z [20] 许静, 尹晓慧, 张光毅. GABAA受体激活BDNF—TrkB—ERK信号通路在脑缺血中的作用[J]. 徐州医学院学报, 2011, 31(7): 441-444. doi: 10.3969/j.issn.1000-2065.2011.07.003 [21] Untereiner A, Xu J, Bhattacharjee A, et al. γ-aminobutyric acid stimulates β-cell proliferation through the mTORC1/p70S6K pathway, an effect amplified by Ly49, a novel γ-aminobutyric acid type A receptor positive allosteric modulator[J]. Diabetes Obes Metab, 2020, 22(11): 2021-2031. doi: 10.1111/dom.14118 [22] Takehara A, Hosokawa M, Eguchi H, et al. Gamma-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor pi subunit[J]. Cancer Res, 2007, 67(20): 9704-9712. doi: 10.1158/0008-5472.CAN-07-2099 [23] Gravielle M C. Regulation of GABA(A) receptors induced by the activation of L-type voltage-gated calcium channels[J]. Membranes, 2021, 11(7): 486. doi: 10.3390/membranes11070486 [24] Yu W, Zhu H, Wang Y, et al. Reactive transformation and increased BDNF signaling by hippocampal astrocytes in response to MK-801[J]. PLoS One, 2015, 10(12): e0145651. doi: 10.1371/journal.pone.0145651 [25] Zhu H, Chen M F, Yu W J, et al. Time-dependent changes in BDNF expression of pentylenetetrazole-induced hippocampal astrocytes in vitro[J]. Brain Res, 2012, 1439: 1-6. doi: 10.1016/j.brainres.2011.12.035 [26] Hol E M, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system[J]. Curr Opin Cell Biol, 2015, 32: 121-130. doi: 10.1016/j.ceb.2015.02.004 [27] 黎影, 骆翔. A1/A2型反应性星形胶质细胞的研究进展[J]. 神经损伤与功能重建, 2022, 17(9): 518-520+528. doi: 10.16780/j.cnki.sjssgncj.20210089 [28] Liddelow S A, Guttenplan K A, Clarke L E, et al. Neurotoxic reactive astrocytes are induced by activated microglia[J]. Nature, 2017, 541(7638): 481-487. doi: 10.1038/nature21029 [29] 王慧芳, 陈新茹, 陈梦圆, 等. 基于JAK2/STAT3通路介导的星形胶质细胞A1/A2表型转化探讨桃红四物汤对脑缺血再灌注损伤的保护作用[J]. 中国实验方剂学杂志, 2025, 31(7): 25-34. doi: 10.13422/j.cnki.syfjx.20242001 [30] Chun H, An H, Lim J, et al. Astrocytic proBDNF and tonic GABA distinguish active versus reactive astrocytes in hippocampus[J]. Exp Neurobiol, 2018, 27(3): 155-170. doi: 10.5607/en.2018.27.3.155 [31] Matsutani S, Yamamoto N. Neuronal regulation of astrocyte morphology in vitro is mediated by GABAergic signaling[J]. Glia, 1997, 20(1): 1-9. doi: 10.1002/(SICI)1098-1136(199705)20:1<1::AID-GLIA1>3.0.CO;2-E [32] Newman E A. Glial cell inhibition of neurons by release of ATP[J]. J Neurosci, 2003, 23(5): 1659-1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003 [33] 李毅豪. Caspase-8/NF-κB信号通路在大鼠脑缺血再灌注损伤炎症机制的调控作用研究[D]. 广州: 广州医科大学, 2021. [34] Wang Y, Wang D, Wang K, et al. γ-Aminobutyric acid alleviates Litchi thaumatin-like protein-induced inflammation and reduces gut microbial translocation[J]. Food Sci Hum Wellness, 2024, 13(5): 3043-3053. doi: 10.26599/FSHW.2022.9250251 [35] Balaratnasingam S, Janca A. Brain derived neurotrophic factor: A novel neurotrophin involved in psychiatric and neurological disorders[J]. Pharmacol Ther, 2012, 134(1): 116-124. doi: 10.1016/j.pharmthera.2012.01.006 [36] Porcher C, Hatchett C, Longbottom R E, et al. Positive feedback regulation between gamma-aminobutyric acid type A (GABA(a)) receptor signaling and brain-derived neurotrophic factor (BDNF) release in developing neurons[J]. J Biol Chem, 2011, 286(24): 21667-21677. doi: 10.1074/jbc.M110.201582 -





 下载:
下载: