The Predictive Value of Gut Metabolite Levels for Myocardial Injury and Prognosis in Patients with Acute Pancreatitis
-
摘要:
目的 探究肠道代谢产物在预测急性胰腺炎(acute pancreatitis,AP)患者发生重症急性胰腺炎(severe acute pancreatitis,SAP)、心肌损伤及不良预后中的价值。 方法 选取2023年4月至2024年4月沧州中西医结合医院收治的80例SAP患者作为重症组研究对象,选取了80例非重症AP患者作为非重症组。比较两组患者入院时血清淀粉酶、脂肪酶、粪便短链脂肪酸(short-chain fatty acids,SCFAs)中乙酸、丙酸、丁酸及总量水平,血清胆汁酸(bile acid,BA)、氧化三甲胺(trimethylamine n-oxide,TMAO)水平,心肌损伤相关指标[肌酸激酶同工酶(creatine kinase isoenzymes,CK-MB)、心肌肌钙蛋白T(cardiac troponin T,cTnT)、N末端B型利钠肽原(N-terminal pro-b-type natriuretic peptide,NT-proBNP)、C反应蛋白(C-reactive protein,CRP)]水平及病情严重程度[急性胰腺炎严重程度床边指数(bedside index for severity in acute pancreatitis,BISAP)、急性生理与慢性健康评估Ⅱ(acute physiology and chronic health evaluation Ⅱ,APACHE Ⅱ)]评分。重症组患者随访30 d,根据预后情况分为生存亚组61例和死亡亚组19例,比较两亚组患者SCFAs总量、BA、TMAO水平。采用Spearman相关性分析所有患者血清淀粉酶、脂肪酶、SCFAs总量、BA、TMAO水平与心肌损伤相关指标、病情严重程度的相关性;多因素Logistic回归分析患者发生SAP的影响因素;采用受试者工作特征(receiver operating characteristic,ROC)曲线分析SCFAs总量、BA、TMAO及联合检测对患者发生SAP的预测价值。 结果 与非重症组患者比较,重症组患者SCFAs中乙酸、丙酸、丁酸及总量水平显著降低(P < 0.01),BA、TMAO、CK-MB、cTnT、NT-proBNP、CRP水平及APACHE Ⅱ、BISAP评分显著升高(P < 0.05);在重症组患者中,死亡亚组较生存亚组的SCFAs总量水平显著降低(P < 0.05),BA、TMAO水平显著升高(P < 0.05)。Spearman分析结果显示,患者CK-MB、cTnT、NT-proBNP、CRP水平及APACHE Ⅱ、BISAP评分与SCFAs总量呈负相关,与BA、TMAO水平呈正相关(P < 0.001);多因素Logistic回归分析显示,SCFAs总量、BA、TMAO是患者发生SAP的独立影响因素(P < 0.05);ROC曲线分析显示,SCFAs总量、BA、TMAO及联合检测评估患者发生SAP的曲线AUC分别为0.951、0.797、0.790、0.974(P < 0.001),三者联合的AUC大于任何单独指标预测,评估效能良好。 结论 SAP患者肠道代谢产物SCFAs、BA、TMAO水平是心肌损伤及预后的独立相关因素。 Abstract:Objective To investigate the value of gut metabolites in predicting the development of severe acute pancreatitis (SAP), myocardial injury, and adverse outcomes in patients with acute pancreatitis (AP). Methods A total of 80 SAP patients admitted to Cangzhou Hospital of Integrated Chinese and Western Medicine from April 2023 to April 2024 were selected as the severe group, and 80 non-severe AP patients were selected as the non-severe group. The levels of serum amylase, lipase, acetic acid, propionic acid, butyric acid, and total short-chain fatty acids (SCFAs) in feces, serum bile acid (BA), trimethylamine n-oxide (TMAO), myocardial injury-related indicators [creatine kinase isoenzymes (CK-MB), cardiac troponin T (cTnT), N-terminal pro-b-type natriuretic peptide (NT-proBNP)], C-reactive protein (CRP), acute physiology and chronic health evaluation II (APACHE II) and bedside index for severity in acute pancreatitis (BISAP) were compared between the two groups at admission. Patients in the severe group were followed up for 30 days and divided into a survival subgroup (n = 61) and a non-survival subgroup (n = 19) based on their prognosis. The levels of total SCFAs, BA, and TMAO were compared between these two subgroups. Spearman correlation analysis was used to analyze the correlation of serum amylase, lipase, total SCFAs, BA, and TMAO levels with myocardial injury-related indicators and disease severity scores in all patients. Multivariate logistic regression analysis was used to identify the influencing factors for the occurrence of SAP. Receiver operating characteristic (ROC) curve analysis was used to evaluate the predictive value of total SCFAs, BA, TMAO, and their combination for the occurrence of SAP. Results Compared with the non-severe group, the severe group had significantly lower levels of acetic acid, propionic acid, butyric acid, and total SCFAs (P < 0.01), and significantly higher levels of BA, TMAO, CK-MB, cTnT, NT-proBNP, CRP, and APACHE II and BISAP scores. Within the severe group, the non-survival subgroup had significantly lower levels of total SCFAs (P < 0.05) and significantly higher levels of BA and TMAO (P < 0.05) compared to the survival subgroup. Spearman analysis showed that the levels of CK-MB, cTnT, NT-proBNP, CRP, and the APACHE II and BISAP scores were negatively correlated with total SCFAs levels and positively correlated with BA and TMAO levels (P < 0.001). Multivariate logistic regression analysis revealed that total SCFAs, BA, and TMAO were independent influencing factors for the occurrence of SAP (P < 0.05). ROC curve analysis showed that the area under the curve (AUC) for total SCFAs, BA, TMAO, and their combination in predicting the occurrence of SAP were 0.951, 0.797, 0.790, and 0.974, respectively (P < 0.001). The AUC for the combination of the three markers was larger than that of any single marker, indicating good predictive efficacy. Conclusion The levels of gut metabolites SCFAs, BA, and TMAO in SAP patients are independent factors associated with myocardial injury and prognosis. -
Key words:
- Severe acute pancreatitis /
- Gut metabolites /
- Myocardial injury /
- Prognosis
-
表 1 两组患者的一般资料比较 [($ \bar x \pm s $)/n(%)]
Table 1. Comparison of general data between the two groups [($ \bar x \pm s $)/n(%)]
组别 SAP组(n=80) AP组(n=80) t/χ2 P 性别 男 43(53.75) 39(48.75) 0.400 0.527 女 37(46.25) 41(51.25) 年龄(岁) 58.19 ± 8.63 58.98 ± 10.05 −0.532 0.320 BMI(kg/m2) 23.04 ± 2.96 23.80 ± 2.92 −1.635 0.797 病因 胆源性 47(58.75) 53(66.25) 1.182 0.757 酒精性 19(23.75) 17(21.25) 血脂性 9(11.25) 6(7.5) 其他 5(6.25) 4(5.0) 合并症 高血压 20(25.00) 17(21.25) 0.316 0.574 糖尿病 9(11.25) 7(8.75) 0.278 0.598 高脂血症 12(15.00) 8(10.00) 0.914 0.339 表 2 两组患者粪便SCFAs及血清淀粉酶、脂肪酶、BA、TMAO水平比较($ \bar x \pm s $,n = 80)
Table 2. Comparison of fecal SCFAs and serum amylase,lipase,BA,and TMAO levels between the two patient groups ($ \bar x \pm s $,n = 80)
组别 SCFAs(μg/g) BA(μmol/L) TMAO(μmol/L) 淀粉酶(U/L) 脂肪酶(U/L) 乙酸 丙酸 丁酸 总量 SAP组 3.09 ± 0.62 3.71 ± 0.76 12.25 ± 1.24 19.05 ± 1.60 4.21 ± 0.84 3.67 ± 0.85 859.24 ± 55.17 373.38 ± 19.41 AP组 4.69 ± 1.03 5.18 ± 1.18 14.26 ± 1.73 24.13 ± 2.55 3.31 ± 0.63 2.82 ± 0.51 575.27 ± 34.75 285.89 ± 14.39 t −11.899 −9.350 −8.456 −15.082 7.709 7.700 38.955 32.395 P <0.001* <0.001* <0.001* <0.001* 0.007* <0.001* <0.001* 0.039* *P < 0.05。 表 3 两组患者心肌损伤相关指标水平及病情严重程度评分比较($ \bar x \pm s $,n = 80)
Table 3. Comparison of myocardial injury-related indices and disease-severity scores between the two patient groups ($ \bar x \pm s $,n = 80)
组别 心肌损伤(ng/mL) 病情严重程度(分) CK-MB cTnT NT-proBNP CRP APACHE Ⅱ BISAP SAP组 11.30 ± 2.37 0.33 ± 0.05 1.41 ± 0.15 2.80 ± 0.48 5.03 ± 0.76 14.66 ± 1.84 AP组 6.14 ± 0.82 0.19 ± 0.03 0.77 ± 0.09 0.85 ± 0.11 2.05 ± 0.50 4.39 ± 0.83 t 18.492 21.876 32.385 35.454 29.167 45.459 P <0.001* <0.001* <0.001* <0.001* 0.001* <0.001* *P < 0.05。 表 4 患者SCFAs总量、BA、TMAO与心肌损伤相关指标及病情严重程度评分的相关性分析
Table 4. Correlation analysis of total fecal SCFAs,BA,and TMAO with myocardial injury-related indices and disease-severity scores
项目 SCFAs总量 BA TMAO r P r P r P 淀粉酶 −0.666 <0.001* 0.420 <0.001* 0.443 <0.001* 脂肪酶 −0.659 <0.001* 0.416 <0.001* 0.459 <0.001* CK-MB −0.655 <0.001* 0.392 <0.001* 0.417 <0.001* cTnT −0.696 <0.001* 0.451 <0.001* 0.459 <0.001* NT-proBNP −0.673 <0.001* 0.467 <0.001* 0.374 <0.001* CRP −0.682 <0.001* 0.453 <0.001* 0.389 <0.001* APACHE Ⅱ −0.709 <0.001* 0.429 <0.001* 0.444 <0.001* BISAP −0.655 <0.001* 0.446 <0.001* 0.453 <0.001* *P < 0.05。 表 5 患者发生SAP影响的多因素Logistic回归分析
Table 5. Multivariate logistic regression analysis of factors influencing SAP occurrence
因素 β SE Waldχ2 P OR 95%CI SCFAs总量 1.049 0.205 26.045 <0.001* 2.854 1.908~4.269 BA −1.831 0.542 11.432 0.001* 0.160 0.055~0.463 TMAO −1.359 0.565 5.788 0.016* 0.257 0.085~0.777 *P < 0.05。 表 6 患者肠道代谢产物水平预测发生SAP的诊断效能
Table 6. Diagnostic performance of gut microbial metabolite levels in predicting SAP
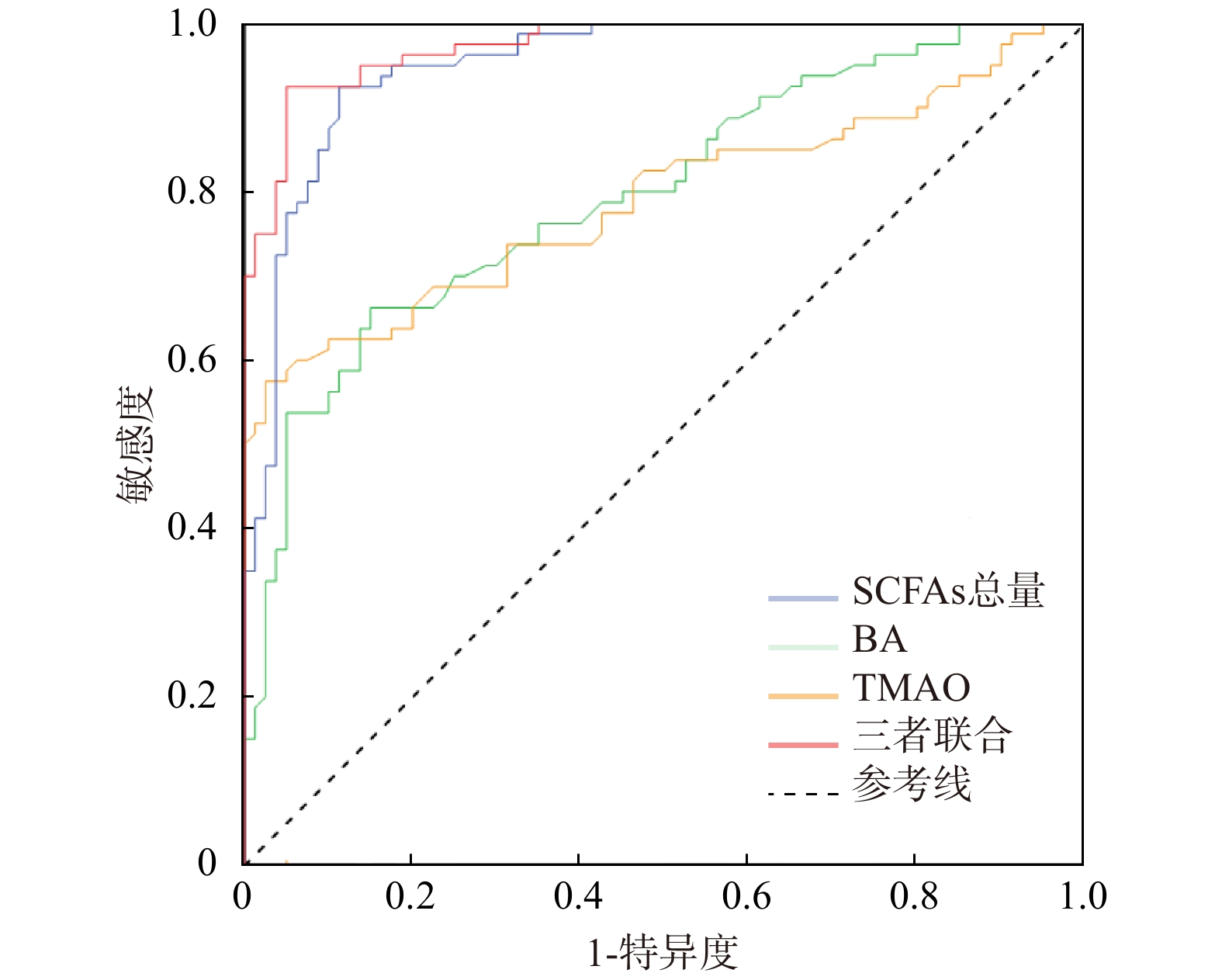
诊断指标 AUC 95%CI 最佳截断值 敏感度 特异度 约登指数 P SCFAs总量 0.951 0.920~0.982 21.350 0.925 0.887 0.812 <0.001* BA 0.797 0.729~0.865 3.850 0.663 0.850 0.513 <0.001* TMAO 0.790 0.717~0.862 3.690 0.575 0.975 0.550 <0.001* 三者联合 0.974 0.955~0.933 0.443 0.925 0.950 0.875 <0.001* *P < 0.05。 表 7 SAP患者中生存亚组与死亡亚组SCFAs、BA、TMAO水平比较
Table 7. Comparison of SCFAs,BA,and TMAO levels between survival and non-survival subgroups in SAP patients
组别 SCFAs总量(μg/g) BA(μmol/L) TMAO(μmol/L) 生存亚组 19.58 ± 1.41 3.88 ± 0.63 3.34 ± 0.68 死亡亚组 17.38 ± 0.87 5.26 ± 0.47 4.72 ± 0.30 t 6.409 −8.806 −8.484 P 0.013* 0.014* <0.001* *P < 0.05。 -
[1] 金海港, 蒋桔红, 朱仲鑫. 1990—2019年中国胰腺炎疾病负担分析[J]. 肝胆胰外科杂志, 2022, 34(6): 344-348. doi: 10.11952/j.issn.1007-1954.2022.06.006 [2] Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: A review[J]. JAMA, 2021, 325(4): 382-390. doi: 10.1001/jama.2020.20317 [3] Trikudanathan G, Yazici C, Evans Phillips A, et al. Diagnosis and management of acute pancreatitis[J]. Gastroenterology, 2024, 167(4): 673-688. doi: 10.1053/j.gastro.2024.02.052 [4] 丁滨, 戴志强, 滕春霞, 等. 大数据解析急诊入院患者疾病谱特征—基于169家综合性医院2015至2017年591万份住院病案首页数据[J]. 中国急救医学, 2019, 39(2): 179-183. [5] Luo Y, Li Z, Ge P, et al. Comprehensive mechanism, novel markers and multidisciplinary treatment of severe acute pancreatitis-associated cardiac injury - a narrative review[J]. J Inflamm Res, 2021, 14(1): 3145-3169. [6] Chen Y, Cui W, Li X, et al. Interaction between commensal bacteria, immune response and the intestinal barrier in inflammatory bowel disease[J]. Front Immunol, 2021, 12(2): 761981. [7] Fan H, Liu X, Ren Z, et al. Gut microbiota and cardiac arrhythmia[J]. Front Cell Infect Microbiol, 2023, 13(2): 1147687. [8] 曹锋, 李非, 赵玉沛. 《中国急性胰腺炎诊治指南(2021)》解读[J]. 中国实用外科杂志, 2021, 41(7): 758-761. [9] 邢斌瑜, 申存毅, 林婷, 等. 急性胰腺炎患者血清TFF3、NF⁃κB水平与预后的关系[J]. 分子诊断与治疗杂志, 2024, 16(11): 2103-2106+2111. doi: 10.3969/j.issn.1674-6929.2024.11.023 [10] 舒玉林, 王逸美, 陈小红, 等. BISAP评分、MLR、NAR在急性胰腺炎病情评估与预后中的价值[J]. 现代消化及介入诊疗, 2024, 29(2): 146-150. doi: 10.3969/j.issn.1672-2159.2024.02.006 [11] 李富金, 陈秋兰, 刘兴红, 等. APACHE Ⅱ评分联合肾血管阻力指数及KIM-1对急性肾损伤患者预后的预测价值[J]. 中国现代医学杂志, 2025, 35(15): 85-90. doi: 10.3969/j.issn.1005-8982.2025.15.013 [12] Ammer-Herrmenau C, Antweiler KL, Asendorf T, et al. Gut microbiota predicts severity and reveals novel metabolic signatures in acute pancreatitis[J]. Gut, 2024, 73(3): 485-495. [13] Li H, Xie J, Guo X, et al. Bifidobacterium spp. and their metabolite lactate protect against acute pancreatitis via inhibition of pancreatic and systemic inflammatory responses[J]. Gut Microbes, 2022, 14(1): 2127456. doi: 10.1080/19490976.2022.2127456 [14] 朱悦楠, 李爱春, 沈施恩, 等. 急性胰腺炎基于肠道菌群和衍生代谢物的微环境变化及相关治疗策略[J]. 中华胰腺病杂志, 2024, 24(5): 387-392. doi: 10.3760/cma.j.cn115667-20231103-00055 [15] Wang Z, Liu J, Li F, et al. Mechanisms of qingyi decoction in severe acute pancreatitis-associated acute lung injury via gut microbiota: targeting the short-chain fatty acids-mediated AMPK/NF-ΚB/NLRP3 pathway[J]. Microbiol Spectr, 2023, 11(4): e0366422. doi: 10.1128/spectrum.03664-22 [16] Xu H, Wan J, He W, et al. Albumin infusion may decrease the mortality of hypoalbuminemia patients with severe acute pancreatitis: A retrospective cohort study[J]. BMC Gastroenterol, 2023, 23(1): 195. doi: 10.1186/s12876-023-02801-8 [17] 徐跃元, 杨上文, 潘俊娣. 重症急性胰腺炎患者肠道菌群和代谢产物的变化特征及其与病情的相关性[J]. 中国微生态学杂志, 2022, 34(11): 1334-1337. [18] Hatamnejad M R, Medzikovic L, Dehghanitafti A, et al. Role of gut microbial metabolites in ischemic and non-ischemic heart failure[J]. Int J Mol Sci, 2025, 26(5): 2242. doi: 10.3390/ijms26052242 [19] 徐臣年, 徐登月, 夏麟, 等. 肠道菌群代谢物三甲胺-N-氧化物与心房颤动关系的研究进展[J]. 心脏杂志, 2024, 36(5): 587-591. doi: 10.12125/j.chj.202304066 [20] 罗佳敏, 肖清玄, 陈宇霞, 等. 从“心合小肠”探讨TGF-β/Smads信号通路与慢性心衰肠道微生态的相关性[J]. 湖南中医药大学学报, 2023, 43(3): 400-404. doi: 10.3969/j.issn.1674-070X.2023.03.006 [21] Baginski A M, Farmer N, Baumer Y, et al. Interleukin-8 (IL-8) as a potential mediator of an association between trimethylamine noxide (TMAO) and proprotein convertase subtilisin/kexin type 9 (PCSK9) among african americans at risk of cardiovascular disease[J]. Metabolites, 2022, 12(12): 1196. doi: 10.3390/metabo12121196 [22] 文江艳, 滕藤, 胡敏, 等. 肠道菌群与心力衰竭关系的研究进展[J]. 中华心力衰竭和心肌病杂志, 2023, 7(1): 69-74. doi: 10.3760/cma.j.cn101460-20220210-00006 -





 下载:
下载: