The Effect of Microbial Metabolites on Immune Function Reconstruction of Aids Based on Mitochondrial Dysfunction
-
摘要:
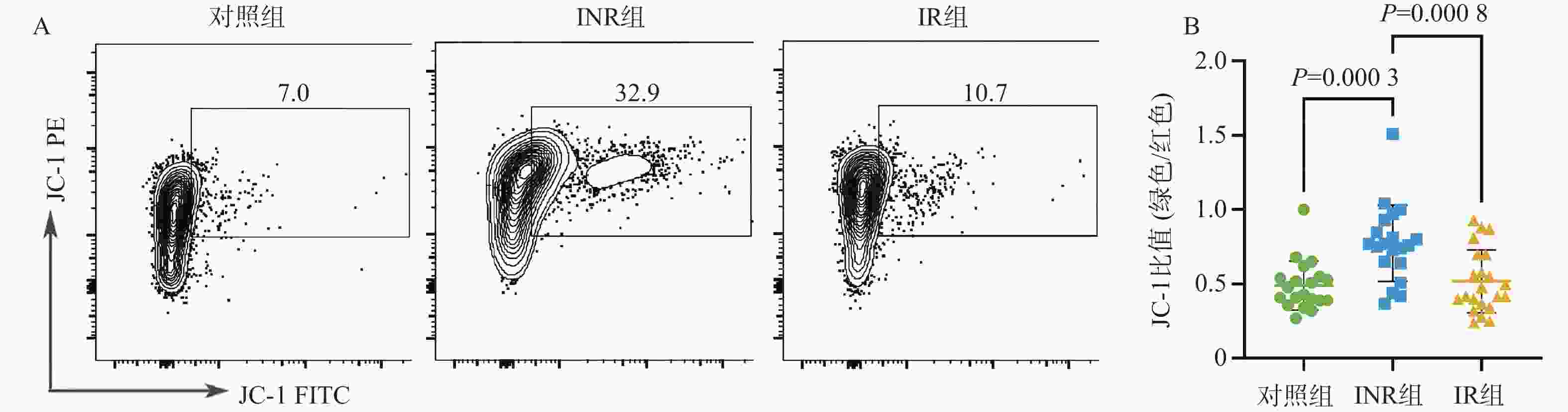
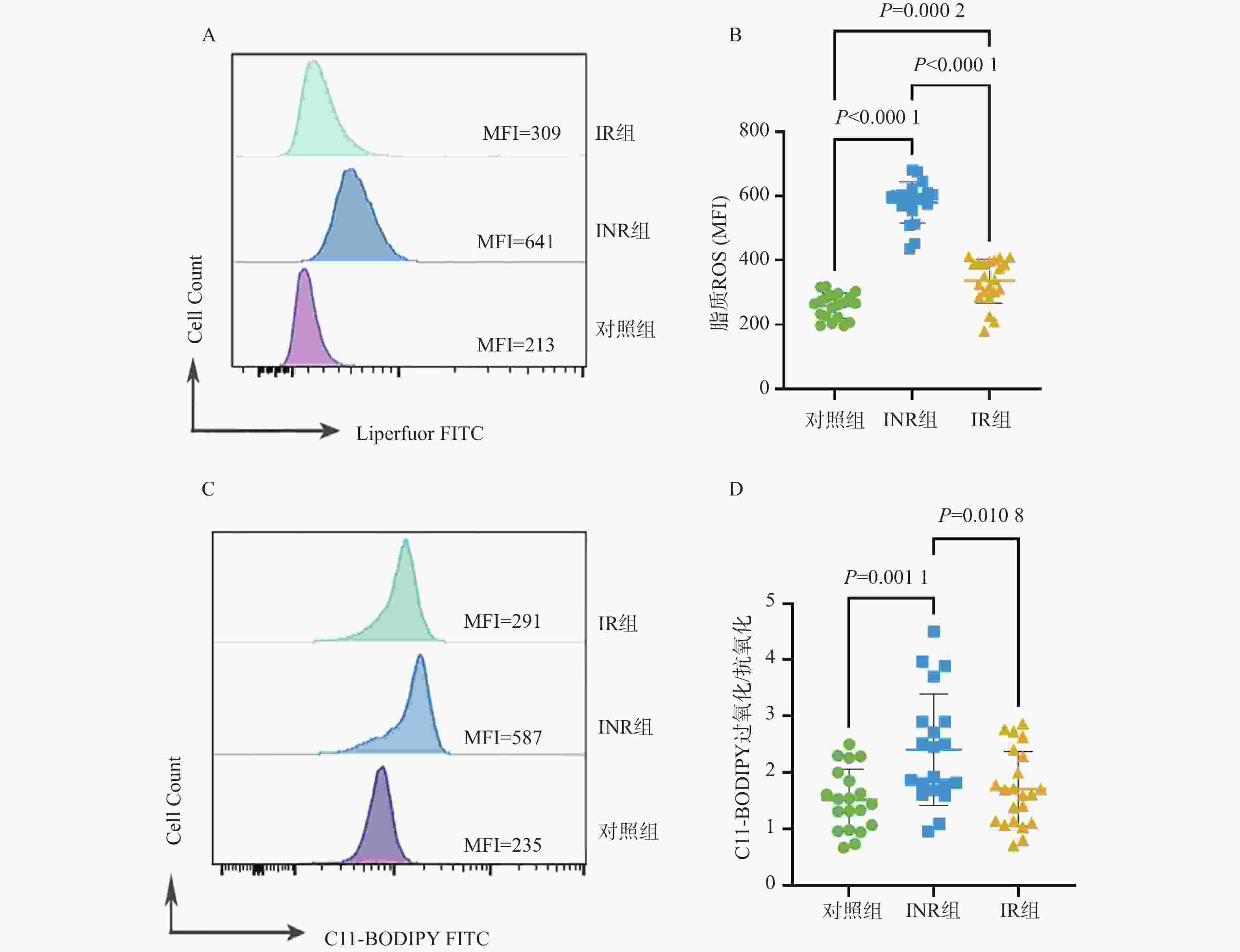
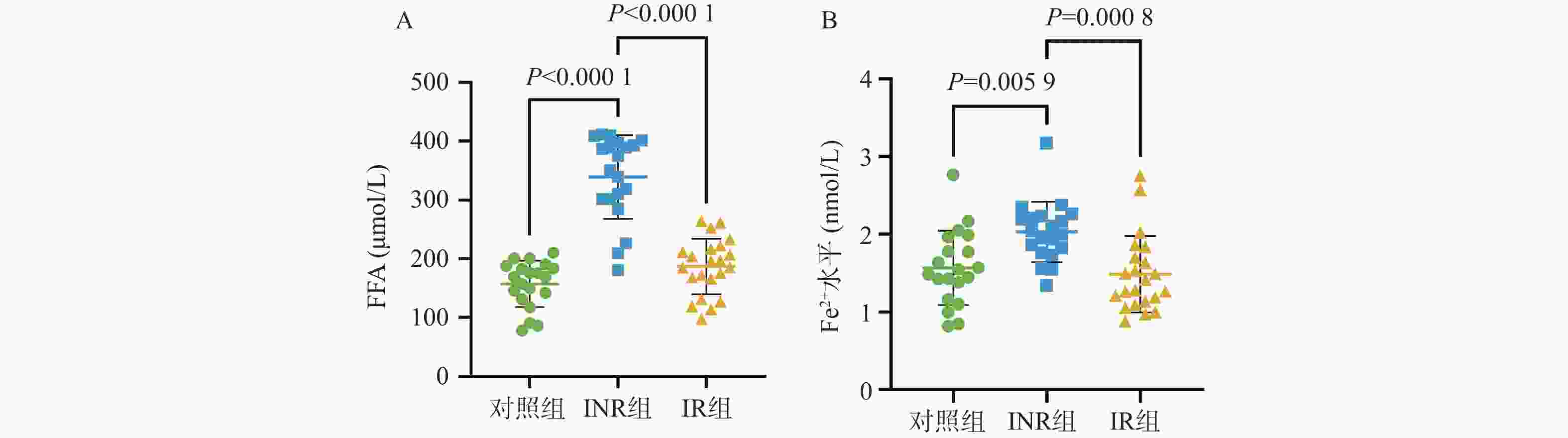
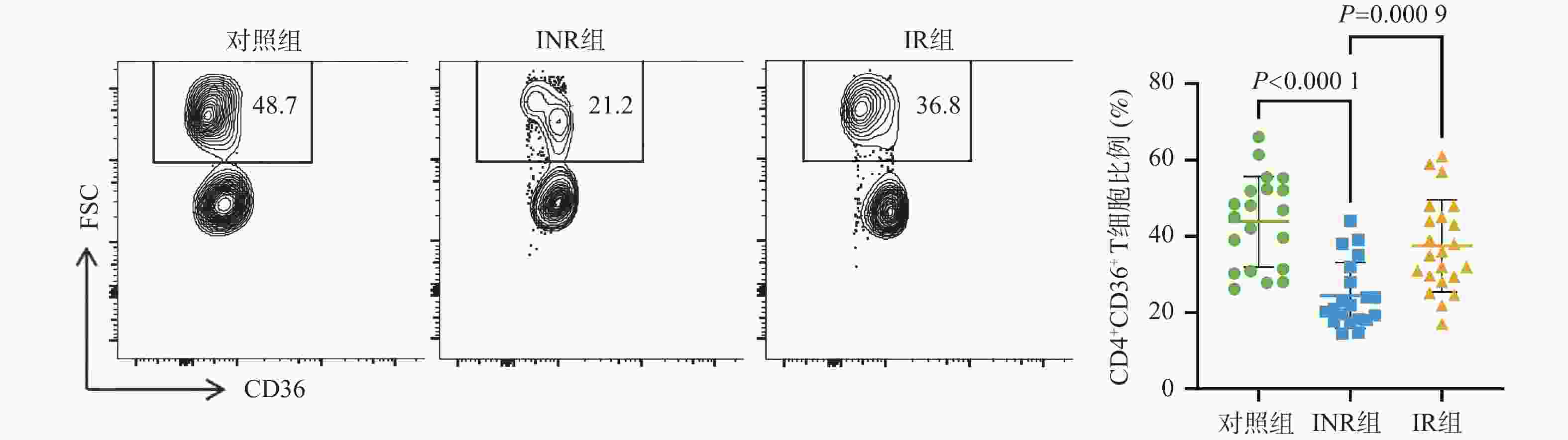
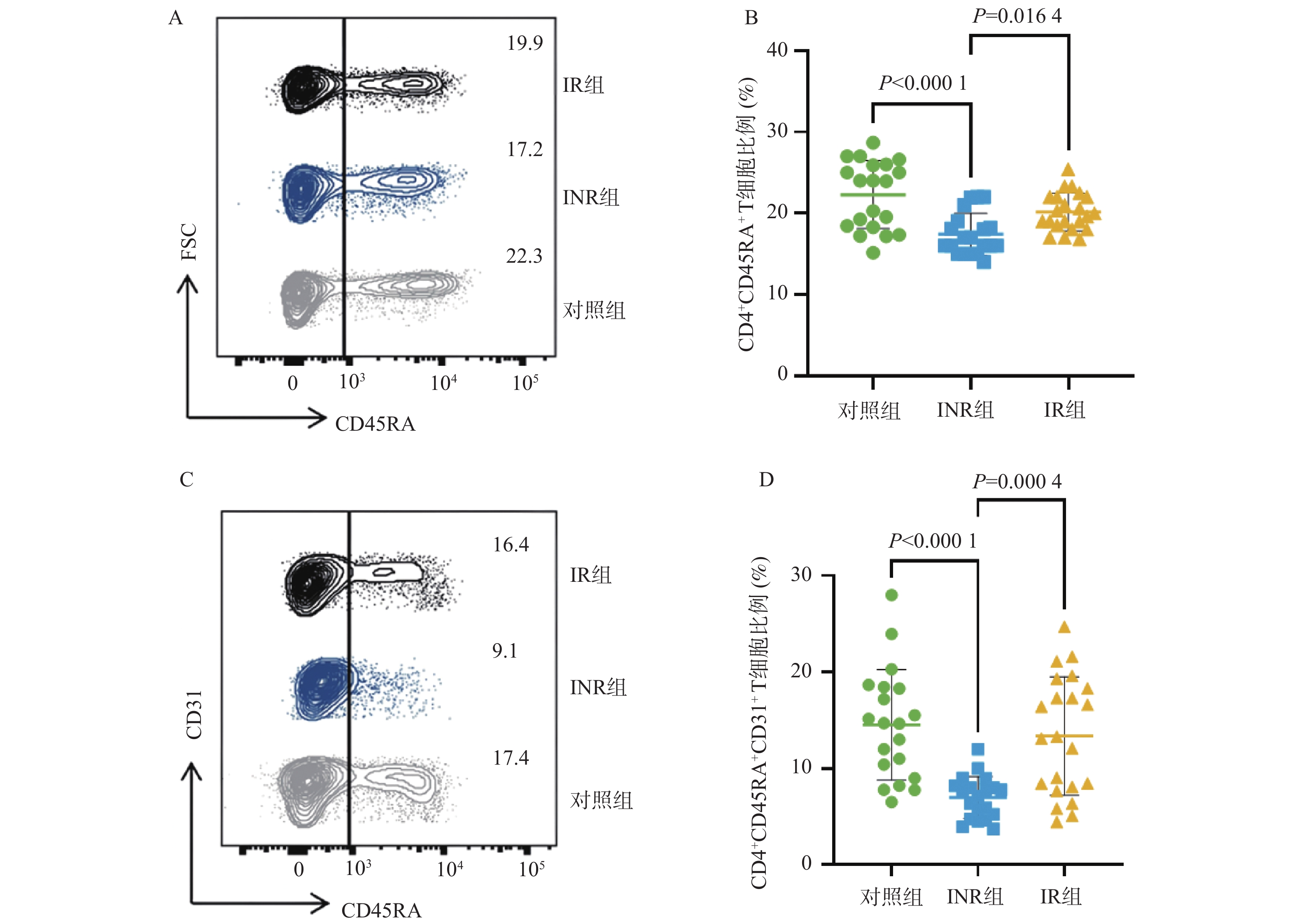
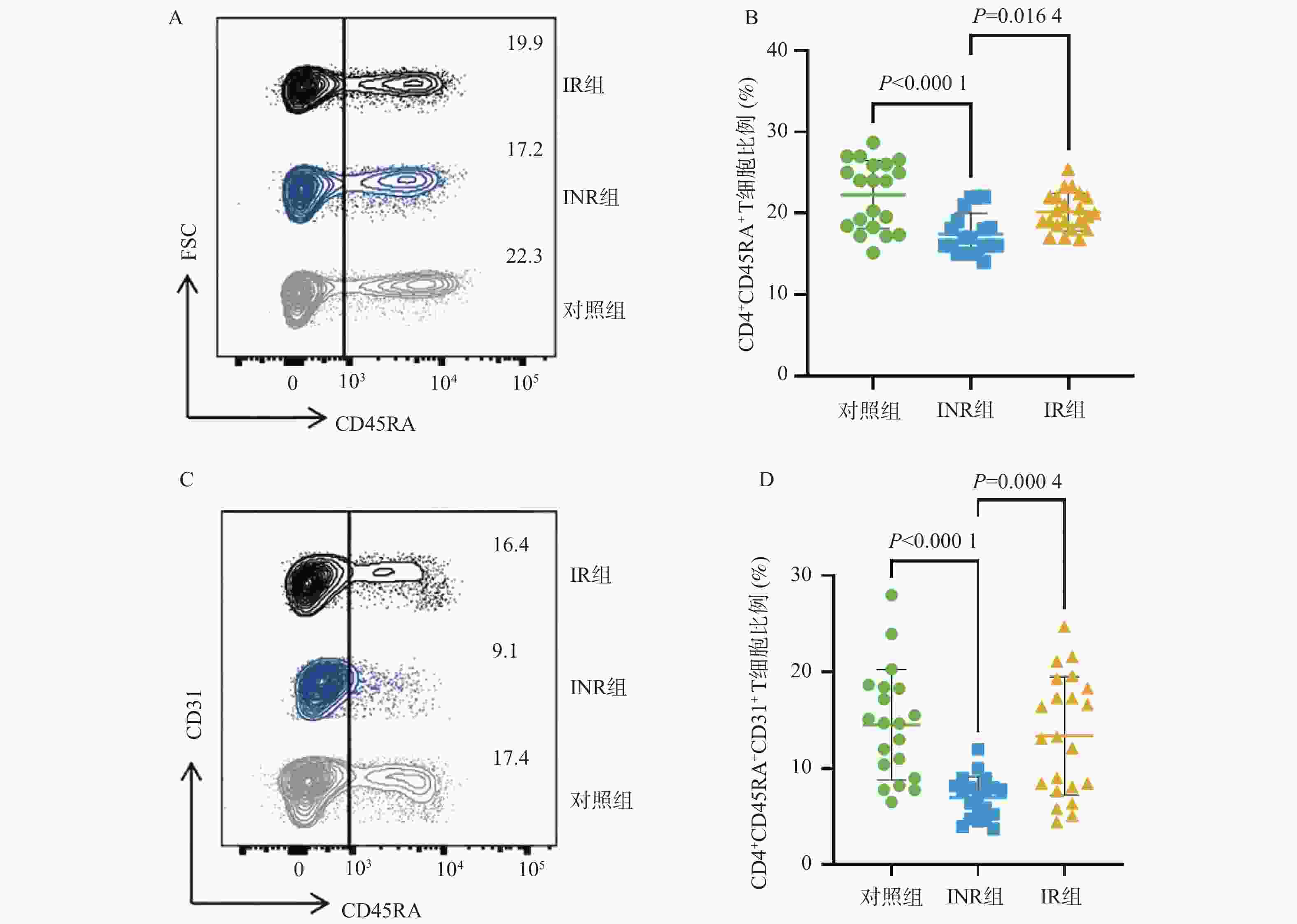
目的 基于线粒体功能障碍探讨菌群代谢产物对艾滋病免疫功能功能重建的影响。 方法 在2023年10月至2024年3月之间招募石家庄市第五医院42名接受4年抗逆转录病毒治疗(antiretroviral therapy,ART)的HIV感染者,包括20名免疫无应答者(immune non-responders,INR)、22名应答者(immune responder,IR)。同时,选择20名性别和年龄匹配的无感染男性接触者作为对照组,其HIV、乙型或丙型肝炎病毒感染均为阴性。采用流式细胞术分析免疫参数。采用游离脂肪酸荧光测定试剂盒和铁比色试剂盒测定血浆中游离脂肪酸(free fatty acid,FFA)和亚铁(Fe2+)水平。 结果 INR组患者的CD4+ T细胞、CD4+CD45RA+ T细胞和CD4+CD45RA+CD31+ T细胞百分比低于对照组和IR组(P < 0.05)。与IR组相比,INR组CD4+T细胞的JC-1比值、脂质反应性氧簇(reactive oxygen species,ROS)水平及C11-BODIPY过氧化/抗氧化升高(P < 0.05)。IR组CD4+T细胞的脂质ROS水平高于对照组(P < 0.05)。INR组患者外周血FFA水平和Fe2+水平高于对照组和IR组(P < 0.05),CD4+CD36+ T细胞百分比低于对照组和IR组(P < 0.05),而对照组和IR组之间的差异无统计学意义(P > 0.05)。 结论 菌群代谢产物FFA升高伴随着CD4+ T细胞上的CD36受体表达降低,导致线粒体功能障碍的发生。 Abstract:objective To explore the effect of microbial metabolites on immune function reconstruction of AIDS based on mitochondrial dysfunction. Methods From October 2023 to March 2024, 42 HIV-infected people who received antiretroviral therapy (ART) for 4 years were recruited, including 20 immune non-responders (INR) and 22 responders (IR). At the same time, 20 non-infected MSM matched by sex and age were selected as the control group, and their HIV, hepatitis B or hepatitis C virus infection were all negative. The immune parameters were analyzed by flow cytometry. The levels of free fatty acid (FFA) and ferrous (Fe2+) in plasma were determined by fluorescence assay kit and iron colorimetric assay kit. Results The percentages of CD4+ T cells, CD4+CD45RA+ T cells and CD4+CD45RA+CD31+ T cells in INR group were lower than those in control group and IR group (P < 0.05). Compared with IR group, the JC-1 ratio of CD4+ T cells, the level of reactive oxygen species (ROS) and the peroxidation/antioxidation of C11-BODIPY in INR group increased (P < 0.05). The lipid ROS level of CD4+ T cells in IR group was higher than that in control group (P < 0.05). The FFA level and Fe2+ level in peripheral blood of patients in INR group were higher than those in control group and IR group (P < 0.05), and the percentage of CD4+CD36+ T cells was lower than that in control group and IR group (P < 0.05), but there was no significant difference between control group and IR group. Conclusion The increase of FFA, a metabolite of flora, is accompanied by the decrease of CD36 receptor expression on CD4+ T cells, leading to mitochondrial dysfunction (P > 0.05). -
Key words:
- HIV /
- Oxidative stress /
- T lymphocytes /
- Mitochondria /
- Antiretroviral therapy /
- Free fatty acids
-
表 1 研究参与者的一般特征 [中位数(Q1,Q3)]
Table 1. General characteristics of study participants [M(Q1,Q3)]
特征 对照组(n=20) INR组(n=20) IR组(n=22) Z/χ2 P 年龄,岁 35(33,38) 39(33,43) 35(31,40) 0.917 0.405 CD4+ T细胞,细胞数/μL 842(535,958)△ 284(225,323) 502(398,711)△ 17.328 <0.001* CD8+ T细胞,细胞数/μL 639(463,870) 581(490,862) 665(505,896) 2.076 0.134 基线CD4+ T细胞,细胞数/μL 163(101,244) 242(193,347) 1.494 0.143 基线HIV RNA,拷贝数/mL 3.544 0.057 ˂105 12 20 ≥105 8 2 治疗方案 - 0.961 TDF + EFV + 3TC 19 22 其他 1 0 ART治疗情况 ART治疗持续时间,年 5.11(4.10,6.52) 5.02(4.15,7.01) 2.021 0.494 成功ART的时间,年 4.63(3.62,6.21) 4.52(3.63,6.52) 1.684 0.297 实验室指标 总胆固醇,mmol/L 4.20(3.65,4.43) 4.17(3.46,4.97) 0.062 0.991 甘油三酯,mmol/L 1.89(1.36,2.77) 1.39(1.01,2.06)△ 4.240 <0.001* HLD,mmol/L 1.03(0.85,1.18) 0.96(0.89,1.26) 1.761 0.086 LDL,mmol/L 2.29(1.98,2.77) 2.41(1.85,3.37) 1.283 0.207 注:*P < 0.05表示组间总体比较有统计学差异;与INR组相比,△P < 0.05。 -
[1] Zaongo S D, Chen Y. Metformin may be a viable adjunctive therapeutic option to potentially enhance immune reconstitution in HIV-positive immunological non-responders[J]. Chin Med J, 2023, 136(18): 2147-2155. doi: 10.1097/CM9.0000000000002493 [2] Gandhi R T, Landovitz R J, Sax P E, et al. Antiretroviral drugs for treatment and prevention of HIV in adults: 2024 recommendations of the international antiviral society-USA panel[J]. Jama, 2025, 333(7): 609. doi: 10.1001/jama.2024.24543 [3] Wang B, Wang Y, Zhang J, et al. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis[J]. Arch Toxicol, 2023, 97(6): 1439-1451. doi: 10.1007/s00204-023-03476-6 [4] Obare L M, Simmons J, Oakes J, et al. CD3+ T-cell: CD14+ monocyte complexes are dynamic and increased with HIV and glucose intolerance[J]. J Immunol, 2025, 214(3): 516-531. doi: 10.1093/jimmun/vkae054 [5] West A L, von Gerichten J, Irvine N A, et al. Fatty acid composition and metabolic partitioning of α-linolenic acid are contingent on life stage in human CD3+ T lymphocytes[J]. Front Immunol, 2022, 13: 1079642. doi: 10.3389/fimmu.2022.1079642 [6] Xiao Q, Yan L, Han J, et al. Metabolism-dependent ferroptosis promotes mitochondrial dysfunction and inflammation in CD4+ T lymphocytes in HIV-infected immune non-responders[J]. eBioMedicine, 2022, 86: 104382. doi: 10.1016/j.ebiom.2022.104382 [7] Barroso S, Guitart-Mampel M, García-García FJ, et al. Metabolic, mitochondrial, and inflammatory effects of efavirenz, emtricitabine, and tenofovir disoproxil fumarate in asymptomatic antiretroviral-naïve people with HIV[J]. Int J Mol Sci, 2024, 25(15): 8418. doi: 10.3390/ijms25158418 [8] Fujii J, Imai H. Oxidative metabolism as a cause of lipid peroxidation in the execution of ferroptosis[J]. Int J Mol Sci, 2024, 25(14): 7544. doi: 10.3390/ijms25147544 [9] Nakanjako D, Nabatanzi R, Ssinabulya I, et al. Chronic immune activation and accelerated immune aging among HIV-infected adults receiving suppressive antiretroviral therapy for at least 12 years in an African cohort[J]. Heliyon, 2024, 10(11): e31910. doi: 10.1016/j.heliyon.2024.e31910 [10] Feng Y Y F, Li Y C, Liu H M, et al. Synthetic lethal CRISPR screen identifies a cancer cell-intrinsic role of PD-L1 in regulation of vulnerability to ferroptosis[J]. Cell Rep, 2024, 43(7): 114477. doi: 10.1016/j.celrep.2024.114477 [11] Morou A, Brunet-Ratnasingham E, Dubé M, et al. Altered differentiation is central to HIV-specific CD4+ T cell dysfunction in progressive disease[J]. Nat Immunol, 2019, 20(8): 1059-1070. doi: 10.1038/s41590-019-0418-x [12] Chen Z, Sun H, Hu T, et al. Sunflower resistance against Sclerotinia sclerotiorum is potentiated by selenium through regulation of redox homeostasis and hormones signaling pathways[J]. Environ Sci Pollut Res, 2022, 29(25): 38097-38109. doi: 10.1007/s11356-021-18125-7 [13] Ma X, Xiao L, Liu L, et al. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability[J]. Cell Metab, 2021, 33(5): 1001-1012. e5. [14] Qian S, Chen X, Wu T, et al. The accumulation of plasma acylcarnitines are associated with poor immune recovery in HIV-infected individuals[J]. BMC Infect Dis, 2021, 21(1): 808. doi: 10.1186/s12879-021-06525-6 [15] Tanase C, Enciu A M, Codrici E, et al. Fatty acids, CD36, thrombospondin-1, and CD47 in glioblastoma: Together and/or separately?[J]. Int J Mol Sci, 2022, 23(2): 604. doi: 10.3390/ijms23020604 [16] Rayasam A, Mottahedin A, Faustino J, et al. Scavenger receptor CD36 governs recruitment of myeloid cells to the blood–CSF barrier after stroke in neonatal mice[J]. J Neuroinflammation, 2022, 19(1): 47. doi: 10.1186/s12974-022-02388-z [17] Ramos-Jiménez A, Zavala-Lira R A, Moreno-Brito V, et al. FAT/CD36 participation in human skeletal muscle lipid metabolism: A systematic review[J]. J Clin Med, 2023, 12(1): 318. [18] Ji C. Molecular factors and pathways of hepatotoxicity associated with HIV/SARS-CoV-2 protease inhibitors[J]. Int J Mol Sci, 2023, 24(9): 7938. doi: 10.3390/ijms24097938 [19] Li Y, Huang X, Yang G, et al. CD36 favours fat sensing and transport to govern lipid metabolism[J]. Prog Lipid Res, 2022, 88: 101193. doi: 10.1016/j.plipres.2022.101193 -





 下载:
下载: