Effects of Dexmedetomidine Combined with Sufentanil on Pain Sensitization and Spinal Inflammatory Response in Rats with Incisional Pain
-
摘要:
目的 探讨右美托咪定(dexmedetomidine,DEX)复合舒芬太尼(sufentanil,SUF)对切口痛大鼠痛觉敏化和脊髓炎性反应的影响。 方法 用大鼠构建切口痛模型,检测每组大鼠的机械缩足阈值(paw withdrawal threshold,PWT)和累计疼痛评分(calculative pain score,CPS)及脊髓组织炎症因子水平。免疫荧光检测离子钙结合衔接分子-1(ionized calcium-binding adapter molecule 1,Iba-1)阳性细胞水平。RT-PCR检测信号调节蛋白α(signal regulatory protein alpha,SIRPα)和整合素相关蛋白(cluster of differentiation 47,CD47)mRNA表达水平。蛋白免疫印迹检测小胶质细胞极化以及SIRPα/CD47通路相关蛋白表达水平。 结果 机械缩足阈值检测及累计疼痛评分评估显示,右美托咪定复合舒芬太尼显著升高大鼠PWT,降低CPS(P < 0.05);酶联免疫吸附试验(enzyme-linked immunosorbent assay,ELISA)结果显示,右美托咪定复合舒芬太尼显著升高大鼠白细胞介素(interleukin,IL)-10水平,减低IL-1β、IL-6水平(P < 0.05);免疫荧光结果显示,右美托咪定复合舒芬太尼显著降低Iba-1阳性细胞数(P < 0.05);RT-PCR结果显示,右美托咪定复合舒芬太尼显著促进大鼠SIRPα和CD47 mRNA表达水平(P < 0.05);蛋白免疫印迹结果显示,右美托咪定复合舒芬太尼显著促进精氨酸酶-1(arginase-1,Arg1和清道夫受体CD163(cluster of differentiation 163,CD163)蛋白表达水平,降低Fcγ受体II(cluster of differentiation 32,CD32)和Fcγ受体III(cluster of differentiation 16,CD16)蛋白表达水平(P < 0.05)。 结论 右美托咪定联合舒芬太尼通过激活SIRPα/CD47通路,抑制小胶质细胞M1极化,减轻大鼠切口痛敏化并降低脊髓炎性因子。 Abstract:Objective To investigate the effects of dexmedetomidine (DEX) combined with sufentanil (SUF) on pain sensitization and spinal inflammatory responses in rats with incisional pain. Methods Incisional pain models were established in rats. The mechanical paw withdrawal threshold (PWT) and cumulative pain score (CPS) of rats in each group, as well as the levels of inflammatory cytokines in spinal cord tissues, were measured. Immunofluorescence was used to detect the level of Iba-1-positive cells. RT-PCR was employed to assess the mRNA expression levels of SIRPα and CD47. Western blotting was conducted to examine the polarization of microglia and the protein expression levels related to the SIRPα/CD47 pathway. Results PWT measurement and CPS assessment demonstrated that dexmedetomidine combined with sufentanil significantly increased the PWT values and decreased the CPS values in rats (P < 0.05). Enzyme-linked immunosorbent assay (ELISA) results revealed that dexmedetomidine combined with sufentanil significantly elevated the levels of interleukin (IL)-10 and reduced the levels of IL-1β and IL-6 in rats (P < 0.05). Immunofluorescence results showed that dexmedetomidine combined with sufentanil significantly decreased the number of Iba-1-positive cells (P < 0.05). RT-PCR results indicated that dexmedetomidine combined with sufentanil significantly promoted the mRNA expression levels of SIRPα and CD47 in rats (P < 0.05). Western blot results demonstrated that dexmedetomidine combined with sufentanil significantly enhanced the protein expression levels of arginase-1 (Arg1) and scavenger receptor cluster of differentiation 163 (CD163), while reducing the protein expression levels of Fcγ receptor II (cluster of differentiation 32, CD32) and Fcγ receptor III (cluster of differentiation 16, CD16) in rats (P < 0.05). Conclusion Dexmedetomidine combined with sufentanil alleviates pain sensitization and reduces spinal inflammatory cytokines in rats with incisional pain by activating the SIRPα/CD47 pathway and inhibiting the M1 polarization of microglia. -
图 1 右美托咪定复合舒芬太尼对切口痛大鼠PWT和CPS的影响($ \bar x \pm s $,n = 7)
A:大鼠PWT的评分;B:大鼠CPS的评分;与Con组相比,*P < 0.05、**P < 0.01;与Model组相比,#P < 0.05、##P < 0.05;与DEX组相比,△P < 0.05、△△P < 0.05;与SUF组相比,▲P < 0.05、▲▲P < 0.05;与DEX+SUF组相比,▽P < 0.05、▽▽P < 0.05。
Figure 1. Effects of dexmedetomidine combined with sufentanil on PWT and CPS in rats with incisional pain ($ \bar x \pm s $,n = 7)
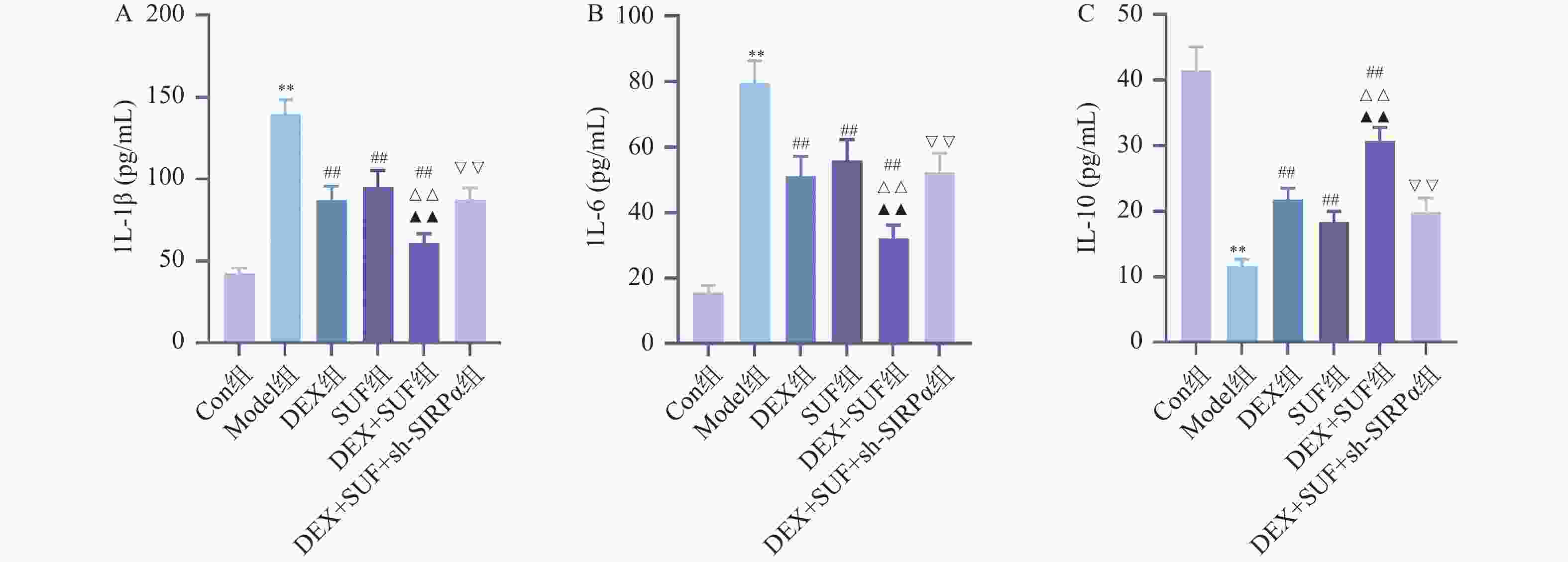
图 2 各组大鼠脊髓组织炎症因子水平的比较($ \bar x \pm s $,n = 7)
A :大鼠脊髓组织IL-1β水平;B:大鼠脊髓组织IL-6水平;C:大鼠脊髓组织IL-10水平;与Con组相比,*P < 0.05、**P < 0.01;与Model组相比,#P < 0.05、##P < 0.05;与DEX组相比,△P < 0.05、△△P < 0.05;与SUF组相比,▲P < 0.05、▲▲P < 0.05;与DEX+SUF组相比,▽P < 0.05、▽▽P < 0.05。
Figure 2. Comparison of inflammatory factors in spinal cord tissue of rats in each group ($ \bar x \pm s $,n = 7)
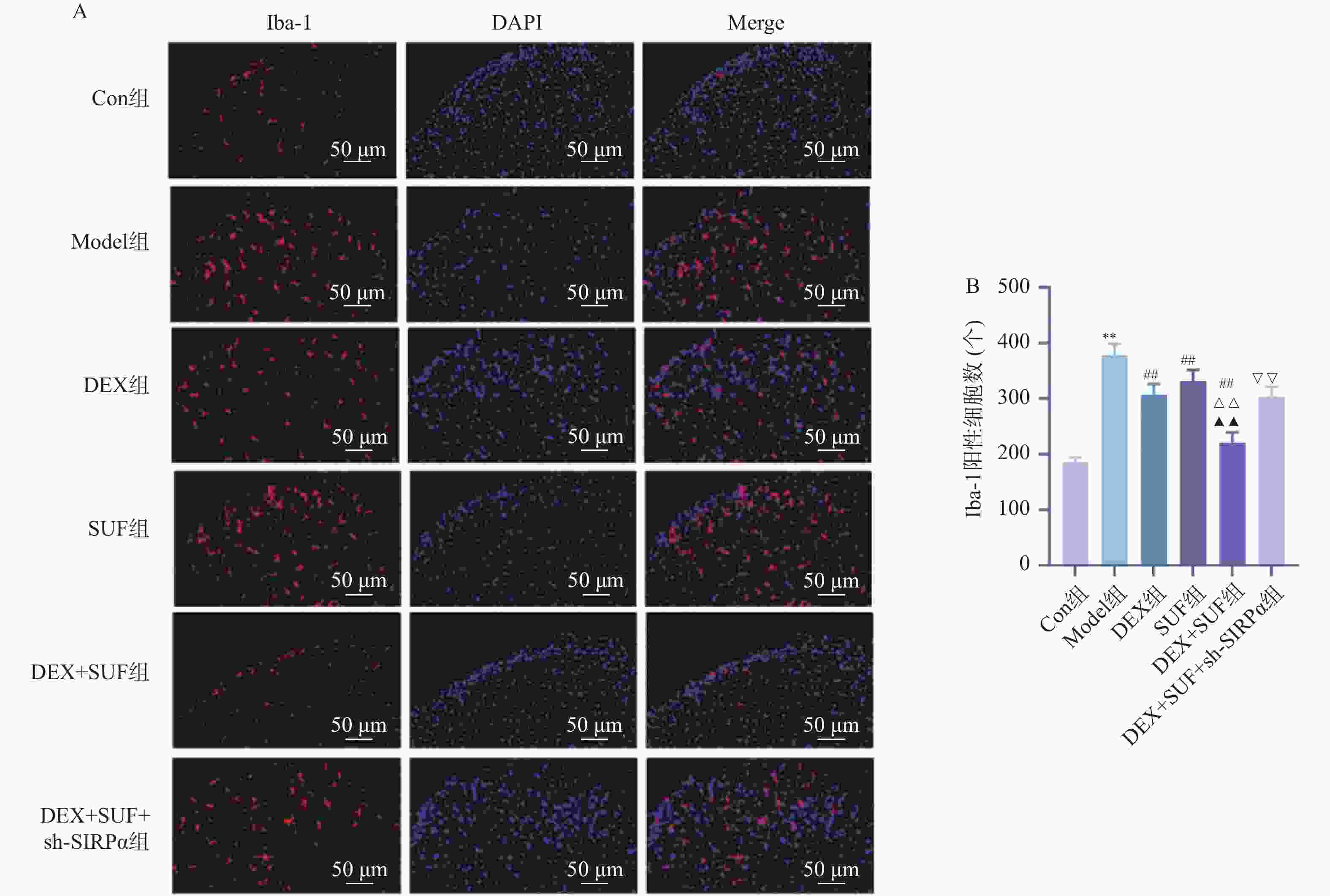
图 3 各组大鼠Iba-1阳性细胞的比较($ \bar x \pm s $,n = 7)
A:免疫荧光检测Iba-1阳性细胞(免疫荧光染色,×200);B:各组大鼠脊髓组织中Iba-1阳性细胞数量;与Con组相比,*P < 0.05、**P < 0.01;与Model组相比,#P < 0.05、##P < 0.05;与DEX组相比,△P < 0.05、△△P < 0.05;与SUF组相比,▲P < 0.05、▲▲P < 0.05;与DEX+SUF组相比,▽P < 0.05、▽▽P < 0.05。
Figure 3. Comparison of Iba-1 positive cells in each group of rats ($ \bar x \pm s $,n = 7)
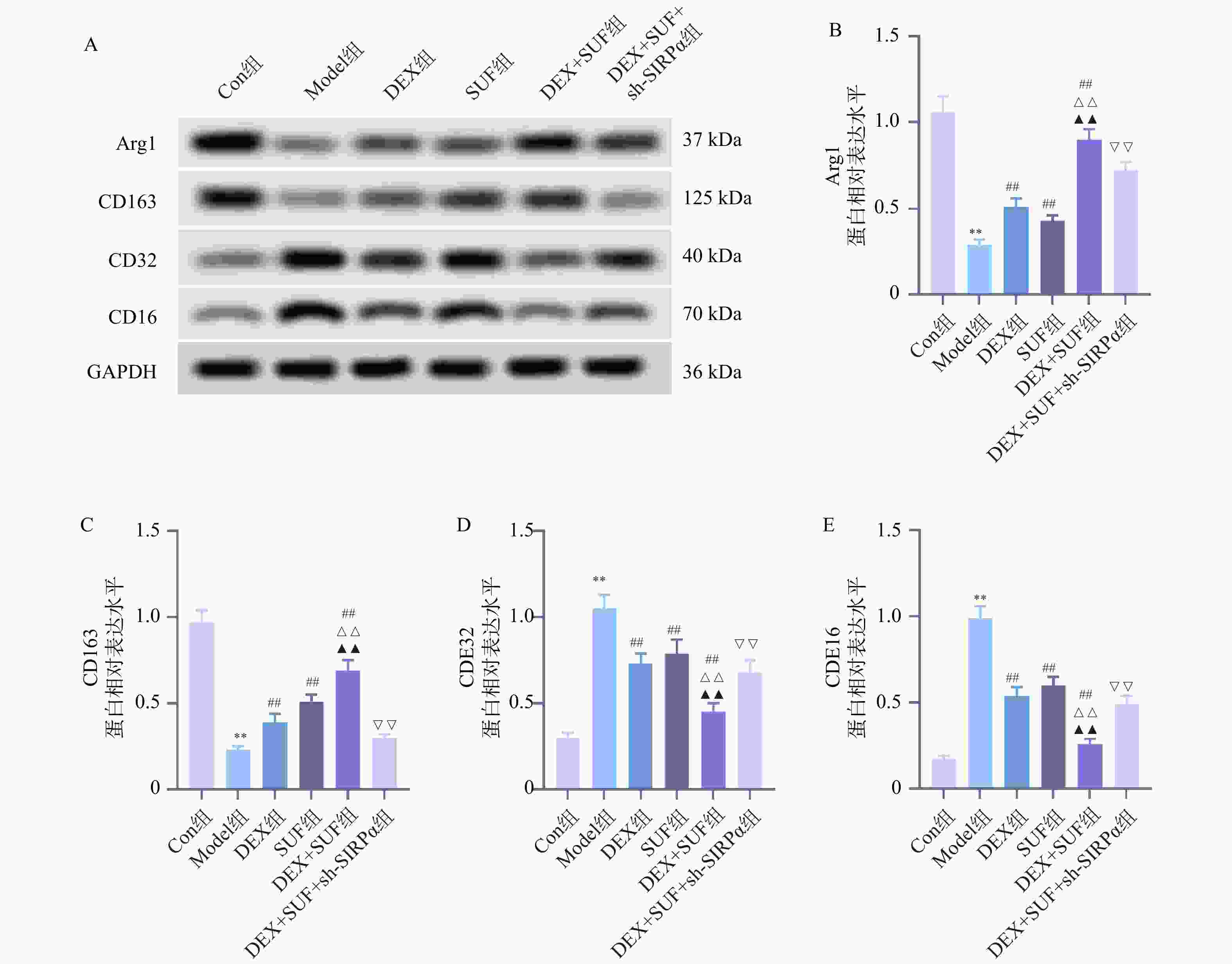
图 4 各组大鼠M1/M2标志型蛋白表达水平的比较($ \bar x \pm s $,n = 7)
A:各组大鼠小胶质细胞M1/M2极化相关蛋白表达;B:大鼠Arg1蛋白表达水平;C:大鼠CD163蛋白表达水平;D:大鼠CD32蛋白表达水平;E:大鼠CD16蛋白表达水平;与Con组相比,*P < 0.05、**P < 0.01;与Model组相比,#P < 0.05、##P < 0.05;与DEX组相比,△P < 0.05、△△P < 0.05;与SUF组相比,▲P < 0.05、▲▲P < 0.05;与DEX+SUF组相比,▽P < 0.05、▽▽P < 0.05。
Figure 4. Comparison of the expression levels of M1/M2 marker proteins in each group of rats ($ \bar x \pm s $,n = 7)
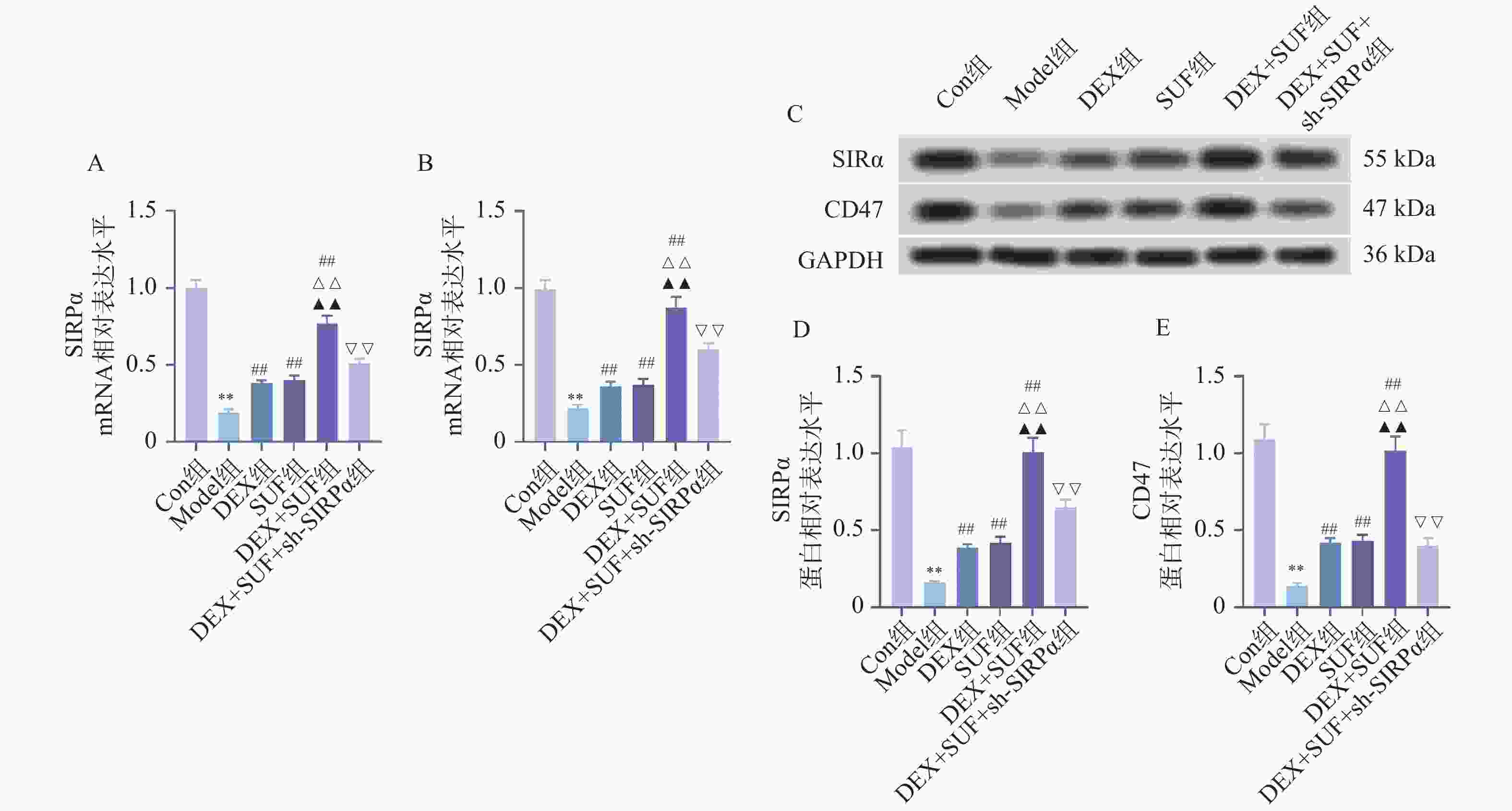
图 5 各组大鼠脊髓组织SIRPα/CD47通路相关蛋白表达水平的比较($ \bar x \pm s $,n = 7)
A:SIRPα mRNA表达水平;B:CD47 mRNA表达水平;C:各组大鼠SIRPα/CD47相关蛋白表达;D:SIRPα 蛋白表达水平;E:CD47 蛋白表达水平;与Con组相比,*P < 0.05、**P < 0.01;与Model组相比,#P < 0.05、##P < 0.05;与DEX组相比,△P < 0.05、△△P < 0.05;与SUF组相比,▲P < 0.05、▲▲P < 0.05;与DEX+SUF组相比,▽P < 0.05、▽▽P < 0.05。
Figure 5. Comparison of the expression levels of SIRPα/CD47 pathway-related proteins in spinal cord tissues of rats in each group ($ \bar x \pm s $,n = 7)
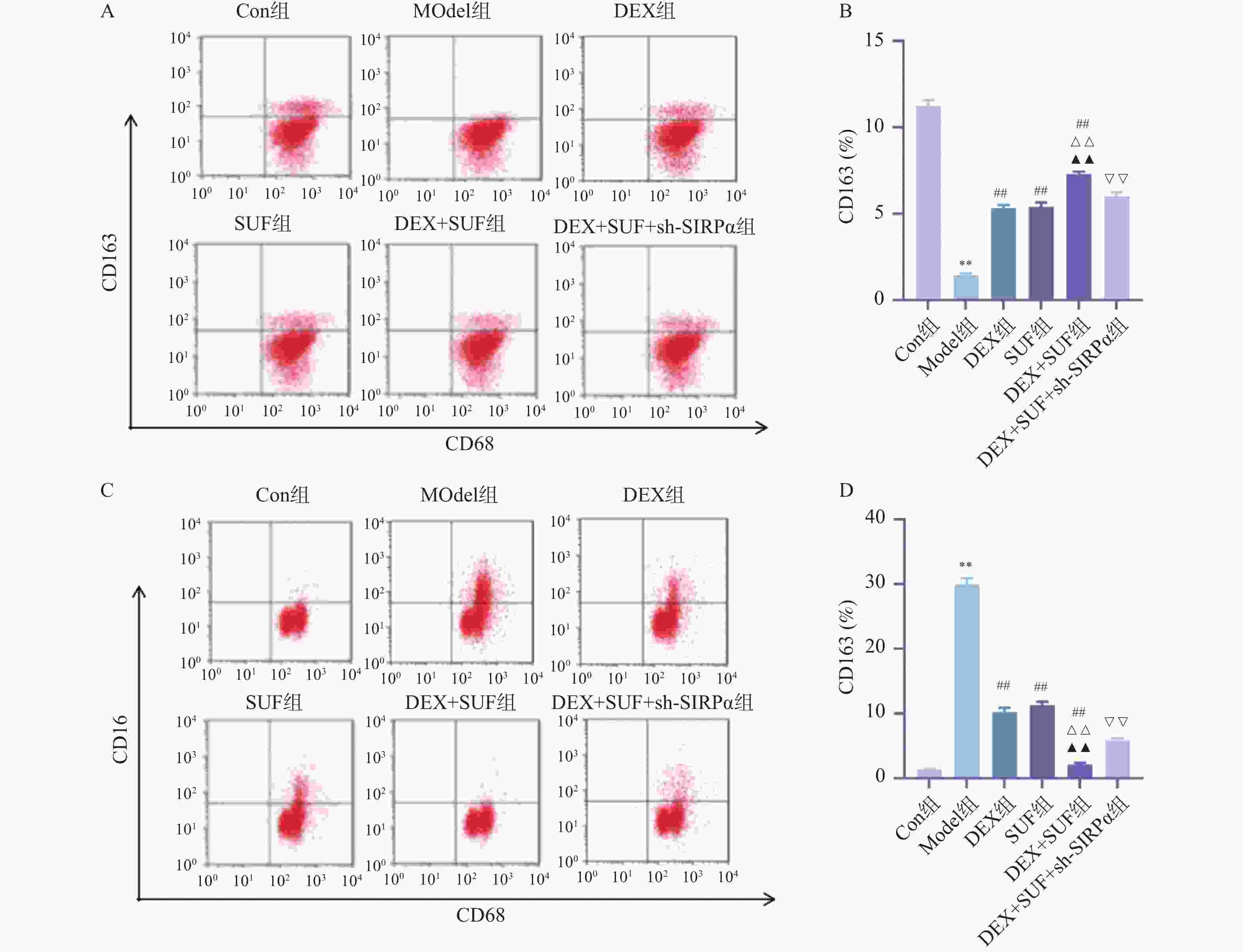
图 7 右美托咪定复合舒芬太尼对原代小胶质细胞极化的影响($ \bar x \pm s $,n = 3)
A:流式细胞术检测CD163细胞比例;B:各组原代小胶质细胞CD163细胞比例;C:流式细胞术检测CD16细胞比例;D:各组原代小胶质细胞CD16细胞比例;与Con组相比,*P < 0.05、**P < 0.01;与Model组相比,#P < 0.05、##P < 0.05;与DEX组相比,△P < 0.05、△△P < 0.05;与SUF组相比,▲P < 0.05、▲▲P < 0.05;与DEX+SUF组相比,▽P < 0.05、▽▽P < 0.05。
Figure 7. Effect of dexmedetomidine combined with sufentanil on polarization of primary microglial cells ($ \bar x \pm s $,n = 3)
表 1 引物序列信息
Table 1. Primer sequence
基因 序列 5'→3' 引物长度(bp) SIRPα F CACTCTGAACTGCACTGTGT 86 R AGATCGGGCTCCGATTTTG CD47 F AATCTCGGTCTCAGACTTGCT 99 R GAAAGCTCAGTCACTTCACAGG GAPDH F CCAAGTACGCTGTGAGTATCAC 89 R CCATGGTTACCGATTAGGGT -
[1] Saito M, Tsukada S, Fujita N, et al. Post-operative pain control following arthroscopic rotator cuff repair: Peri-articular injection versus interscalene brachial plexus block[J]. Int Orthop, 2019, 43(6): 1435-1441. doi: 10.1007/s00264-018-4096-3 [2] Joseph J M, Gori D, Curtin C, et al. Gaps in standardized postoperative pain management quality measures: A systematic review[J]. Surgery, 2022, 171(2): 453-458. doi: 10.1016/j.surg.2021.08.004 [3] Erlenwein J, Emons M I, Petzke F, et al. The effectiveness of an oral opioid rescue medication algorithm for postoperative pain management compared to PCIA: A cohort analysis[J]. Anaesthesist, 2020, 69(9): 639-648. doi: 10.1007/s00101-020-00806-6 [4] 陈方园, 林多茂. 剖宫产术中应用舒芬太尼对卡前列素所诱发不良反应的影响[J]. 中国医药, 2023, 18(1): 91-94. doi: 10.3760/j.issn.1673-4777.2023.01.021 [5] 王国庆. 不同剂量右美托咪定辅助舒芬太尼用于癌痛患者PCIA镇痛的比较[J]. 中国现代医生, 2022, 60(3): 142-145. [6] 江磊, 贾子普, 罗芳. 预先切口浸润氟比洛芬酯对切口痛大鼠术后疼痛及炎症细胞因子的影响[J]. 中国老年学杂志, 2022, 42(14): 3566-3570. doi: 10.3969/j.issn.1005-9202.2022.14.053 [7] 舒洛娃, 王古岩. 右美托咪定及舒芬太尼联合鞘内注射对CCI模型大鼠DRG神经元GABAA激活电流的作用[J]. 中国中西医结合外科杂志, 2021, 27(4): 632-637. doi: 10.3969/j.issn.1007-6948.2021.04.016 [8] 胡荟琴, 李琳, 胡小伟, 等. 槲皮素促进脂多糖诱导的原代小胶质细胞M2型极化及机制研究[J]. 浙江中医药大学学报, 2025, 49(3): 259-271. doi: 10.16466/j.issn1005-5509.2025.03.001 [9] 赵灿, 赵二贤, 张乐. 胰岛素样生长因子1对切口痛大鼠痛觉敏化和脊髓炎性反应的影响[J]. 武汉大学学报(医学版), 2023, 44(5): 533-537. doi: 10.14188/j.1671-8852.2022.0470 [10] 殷梦兰, 刘丹红, 王贝. 右美托咪定复合舒芬太尼用于腹腔镜下子宫全切术后镇痛的临床价值[J]. 麻醉安全与质控, 2024, 8(6): 313-317. doi: 10.3969/j.issn.2096-2681.2024.06.004 [11] 王明安. 右美托咪定复合舒芬太尼术后镇痛对经腹子宫切除术患者睡眠质量的影响[J]. 世界睡眠医学杂志, 2020, 7(10): 1718-1719. doi: 10.3969/j.issn.1004-5805.2017.03.003 [12] 许畅, 俞晨晨, 李桦, 等. 舒芬太尼与右美托咪啶联合用药在大鼠中的药动学及其镇静作用[J]. 中国药理学与毒理学杂志, 2019, 33(1): 63-69. doi: 10.3867/j.issn.1000-3002.2019.01.009 [13] Kaye A D, Chernobylsky D J, Thakur P, et al. Dexmedetomidine in enhanced recovery after surgery (ERAS) protocols for postoperative pain[J]. Curr Pain Headache Rep, 2020, 24(5): 21. doi: 10.1007/s11916-020-00853-z [14] 丁滢燕, 朱文伟. 腹腔镜下子宫全切术舒芬太尼、布托啡诺、右美托咪定联合应用对患者术后认知及免疫球蛋白影响[J]. 中国计划生育学杂志, 2023, 31(6): 1311-1315. doi: 10.3969/j.issn.1004-8189.2023.06.012 [15] Tang C, Hu Y, Zhang Z, et al. Dexmedetomidine with sufentanil in intravenous patient-controlled analgesia for relief from postoperative pain, inflammation and delirium after esophageal cancer surgery[J]. Biosci Rep, 2020, 40(5): BSR20193410. doi: 10.1042/BSR20193410 [16] 邓小力, 袁秀仪, 林福森. 舒芬太尼联合右美托咪定应用于临床麻醉及术后镇痛中的效果分析[J]. 中国现代药物应用, 2025, 19(1): 99-102. doi: 10.14164/j.cnki.cn11-5581/r.2025.01.027 [17] 任晓亮. 舒芬太尼与右美托咪定在术后镇痛中的应用效果研究[J]. 中国实用医药, 2024, 19(3): 112-114. doi: 10.14163/j.cnki.11-5547/r.2024.03.029 [18] Siracusa R, Fusco R, Cordaro M, et al. The protective effects of pre- and post-administration of micronized palmitoylethanolamide formulation on postoperative pain in rats[J]. Int J Mol Sci, 2020, 21(20): 7700. doi: 10.3390/ijms21207700 [19] Zhang J, Chen S, Zhang R, et al. Rapamycin ameliorates inflammatory pain via recovery of autophagy flux mediated by mammalian target of rapamycin (mTOR) signaling pathway in the rat spinal cord[J]. Int J Immunopathol Pharmacol, 2025, 39: 03946320251317284. [20] Jiang W, Zhang L X, Tan X Y, et al. Inflammation and histone modification in chronic pain[J]. Front Immunol, 2023, 13: 1087648. doi: 10.3389/fimmu.2022.1087648 [21] 林玉美, 符明君, 陈基胜. 舒芬太尼对神经病理性疼痛大鼠的镇痛作用及其机制[J]. 中国老年学杂志, 2025, 45(4): 918-922. [22] 艾丽丽, 韩亮, 王昊, 等. 罗哌卡因联合右美托咪定或地塞米松对老年肋间神经阻滞患者的效果及炎症因子的影响[J]. 中国老年学杂志, 2024, 44(10): 2380-2384. [23] Jurga A M, Paleczna M, Kuter K Z. Overview of general and discriminating markers of differential microglia phenotypes[J]. Front Cell Neurosci, 2020, 14: 198. doi: 10.3389/fncel.2020.00198 [24] Hankerd K, McDonough K E, Wang J, et al. Postinjury stimulation triggers a transition to nociplastic pain in mice[J]. Pain, 2022, 163(3): 461-473. doi: 10.1097/j.pain.0000000000002366 [25] Domoto R, Sekiguchi F, Tsubota M, et al. Macrophage as a peripheral pain regulator[J]. Cells, 2021, 10(8): 1881. doi: 10.3390/cells10081881 [26] Yang H, Zhang Y, Duan Q, et al. Dehydrocorydaline alleviates sleep deprivation-induced persistent postoperative pain in adolescent mice through inhibiting microglial P2Y(12) receptor expression in the spinal cord[J]. Mol Pain, 2023, 19: 17448069231216234. [27] Atta A A, Ibrahim W W, Mohamed A F, et al. Microglia polarization in nociplastic pain: Mechanisms and perspectives[J]. Inflammopharmacology, 2023, 31(3): 1053-1067. doi: 10.1007/s10787-023-01216-x [28] Zhang L Q, Gao S J, Sun J, et al. DKK3 ameliorates neuropathic pain via inhibiting ASK-1/JNK/p-38-mediated microglia polarization and neuroinflammation[J]. J Neuroinflammation, 2022, 19(1): 129. doi: 10.1186/s12974-022-02495-x [29] Huang C, Wang X, Wang Y, et al. Sirpα on tumor-associated myeloid cells restrains antitumor immunity in colorectal cancer independent of its interaction with CD47[J]. Nat Cancer, 2024, 5(3): 500-516. doi: 10.1038/s43018-023-00691-z [30] Ding X, Wang J, Huang M, et al. Loss of microglial SIRPα promotes synaptic pruning in preclinical models of neurodegeneration[J]. Nat Commun, 2021, 12(1): 2030. doi: 10.1038/s41467-021-22301-1 [31] Gao X, Yang H, Xiao W, et al. Modified exosomal SIRPα variants alleviate white matter injury after intracerebral hemorrhage via microglia/macrophages[J]. Biomater Res, 2022, 26(1): 67. doi: 10.1186/s40824-022-00311-4 [32] Zhang Z, Mao Y, Huang S, et al. Microglia promote inhibitory synapse phagocytosis in the spinal cord dorsal horn and modulate pain-like behaviors in a murine cancer-induced bone pain model[J]. Anesth Analg, 2024, 139(2): 411-419. doi: 10.1213/ANE.0000000000006824 -






 下载:
下载: