Effects of p16 on the Cell Cycle and Fatty Acid Metabolism of Gallbladder Cancer Cells
-
摘要:
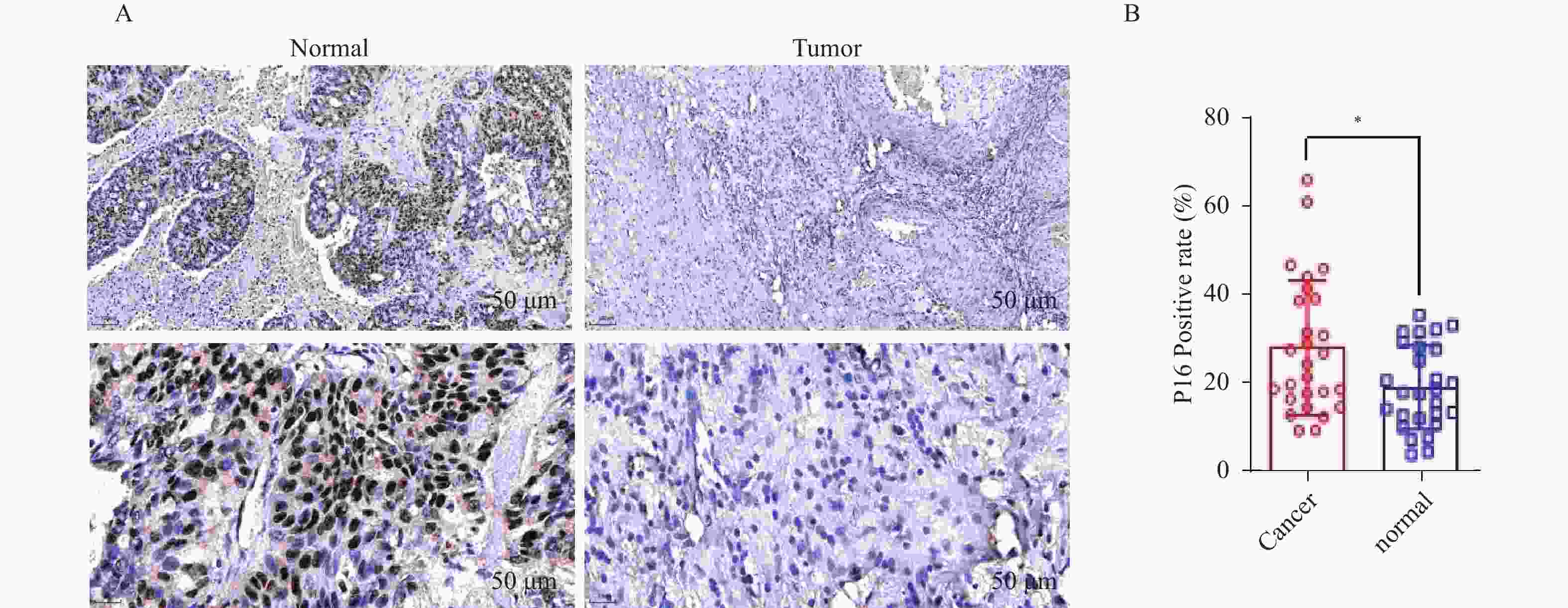
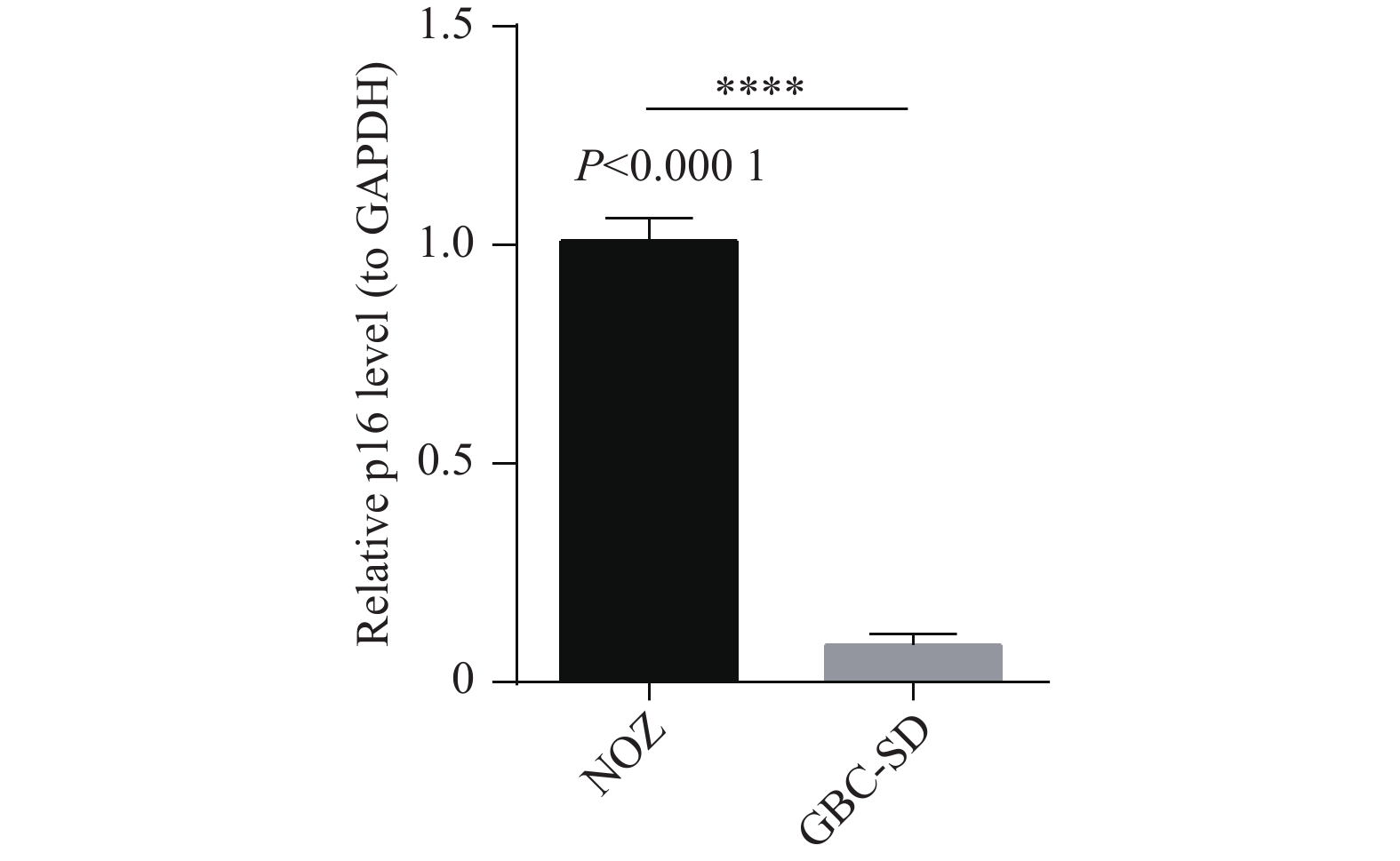
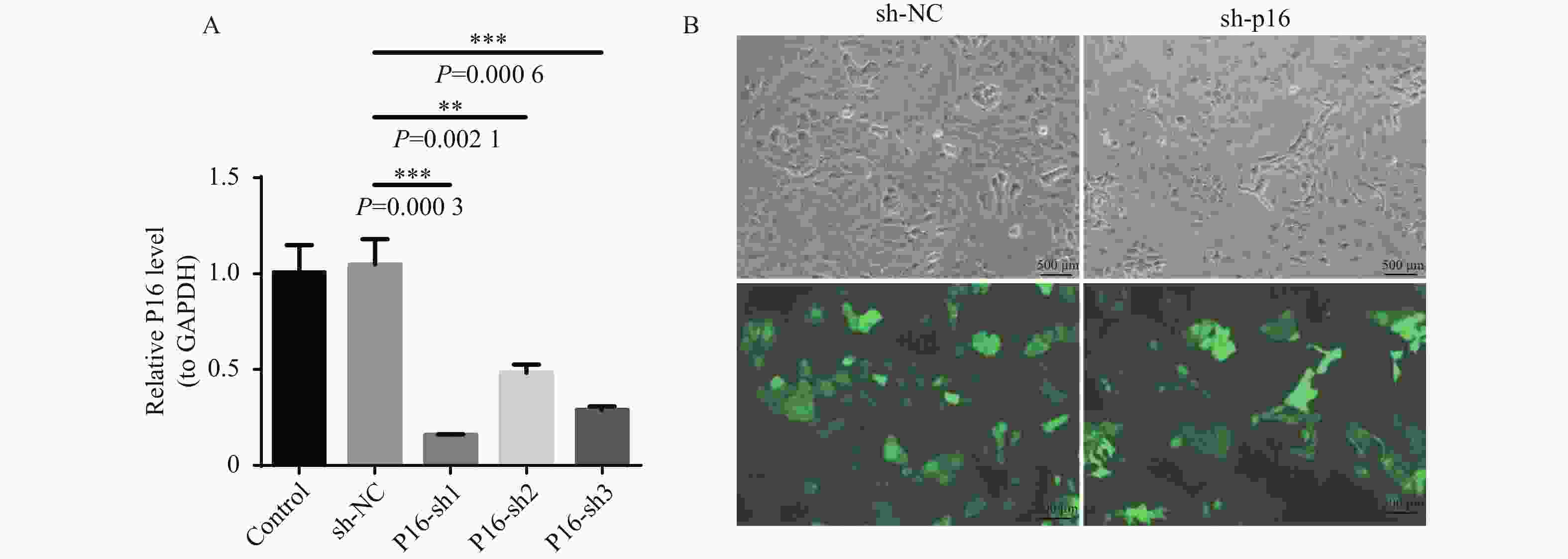
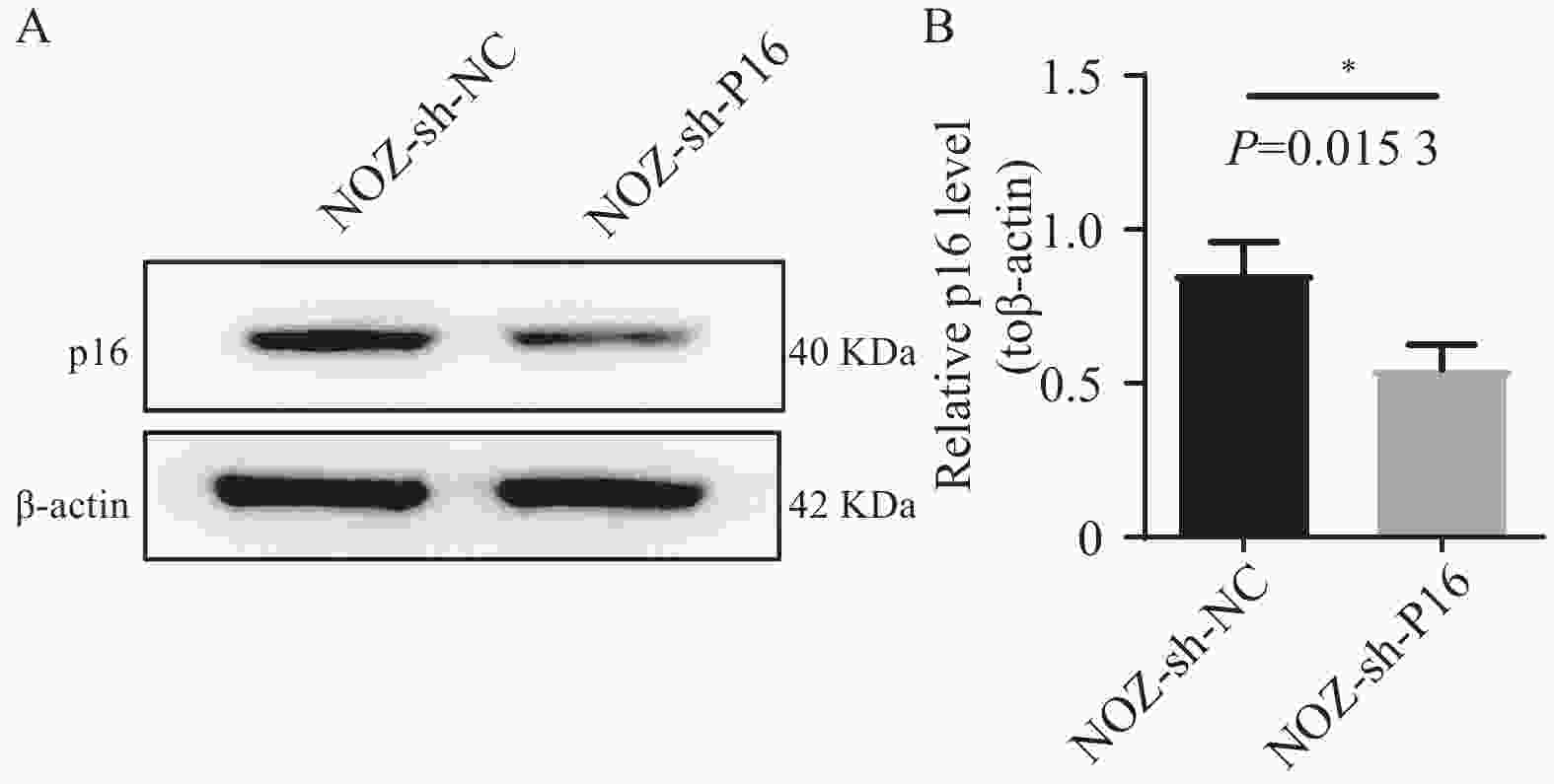
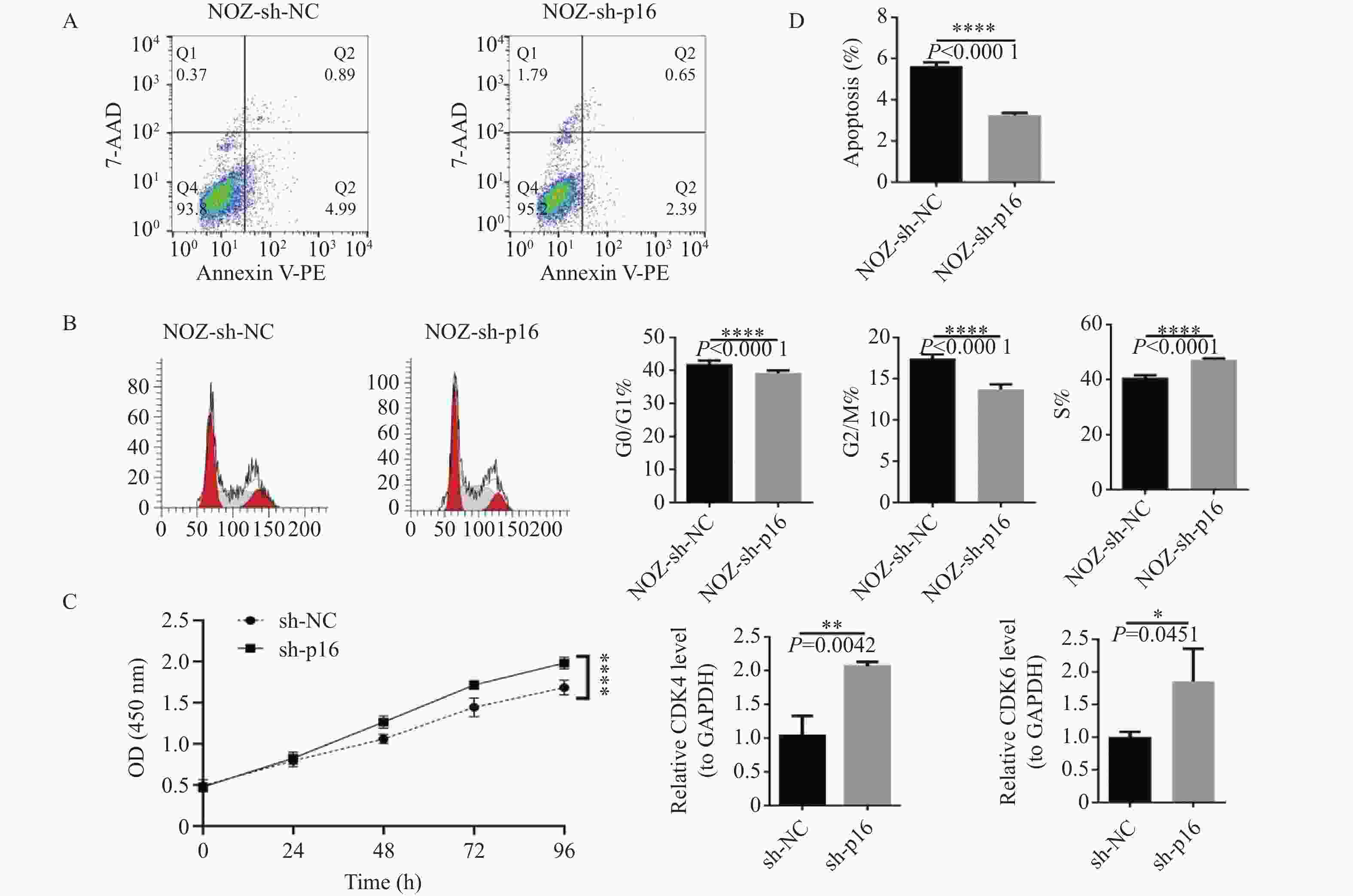
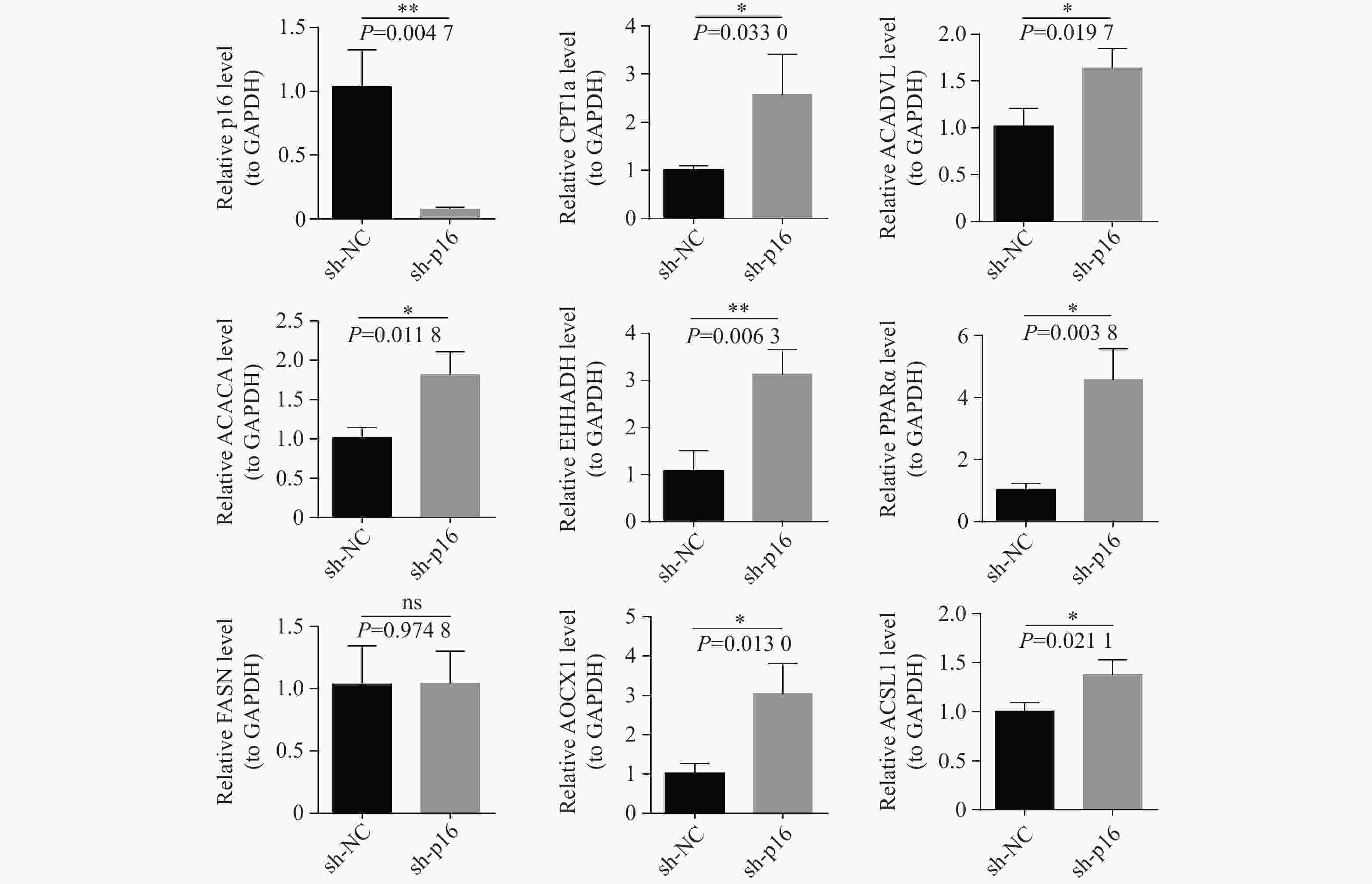
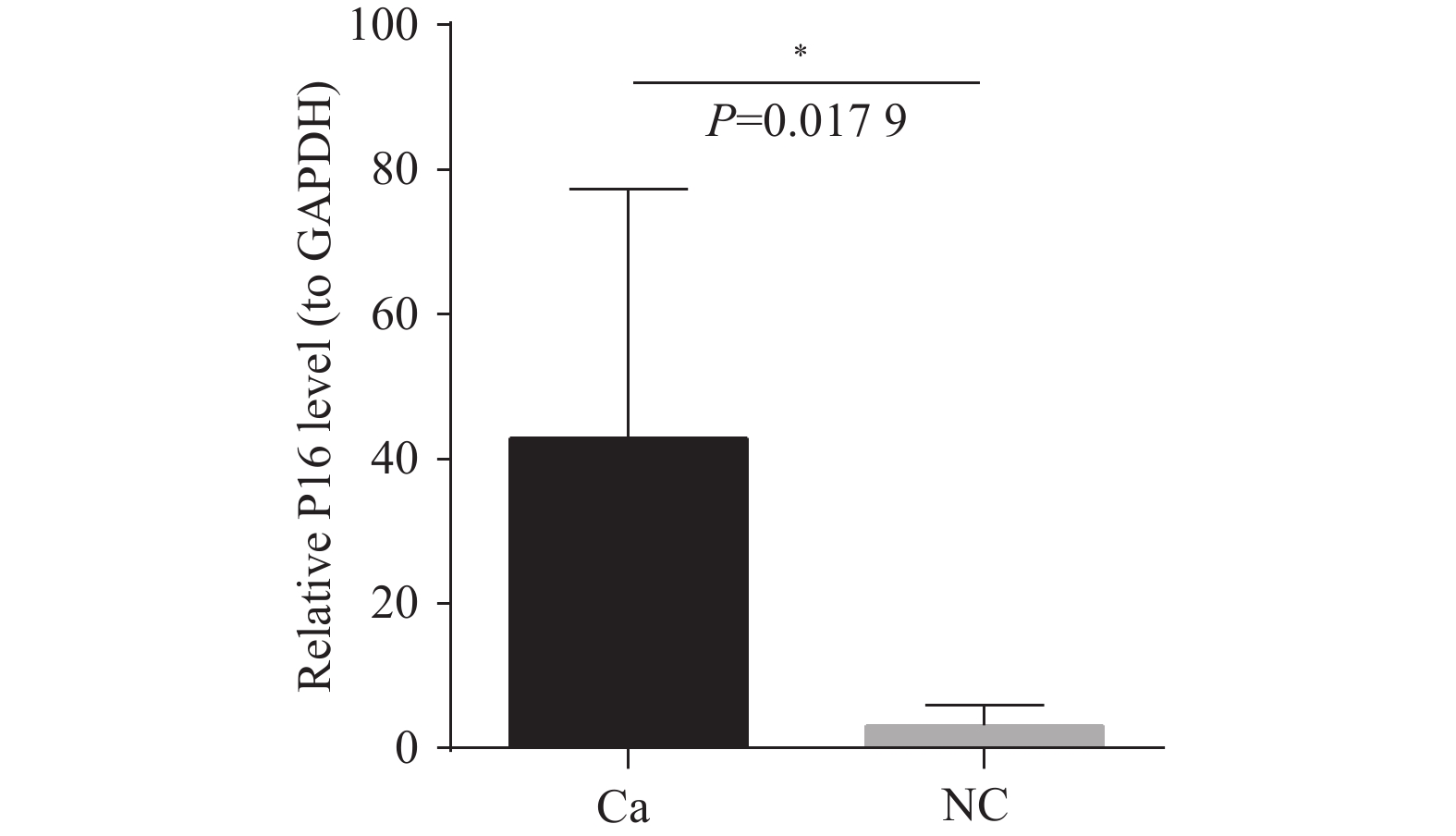
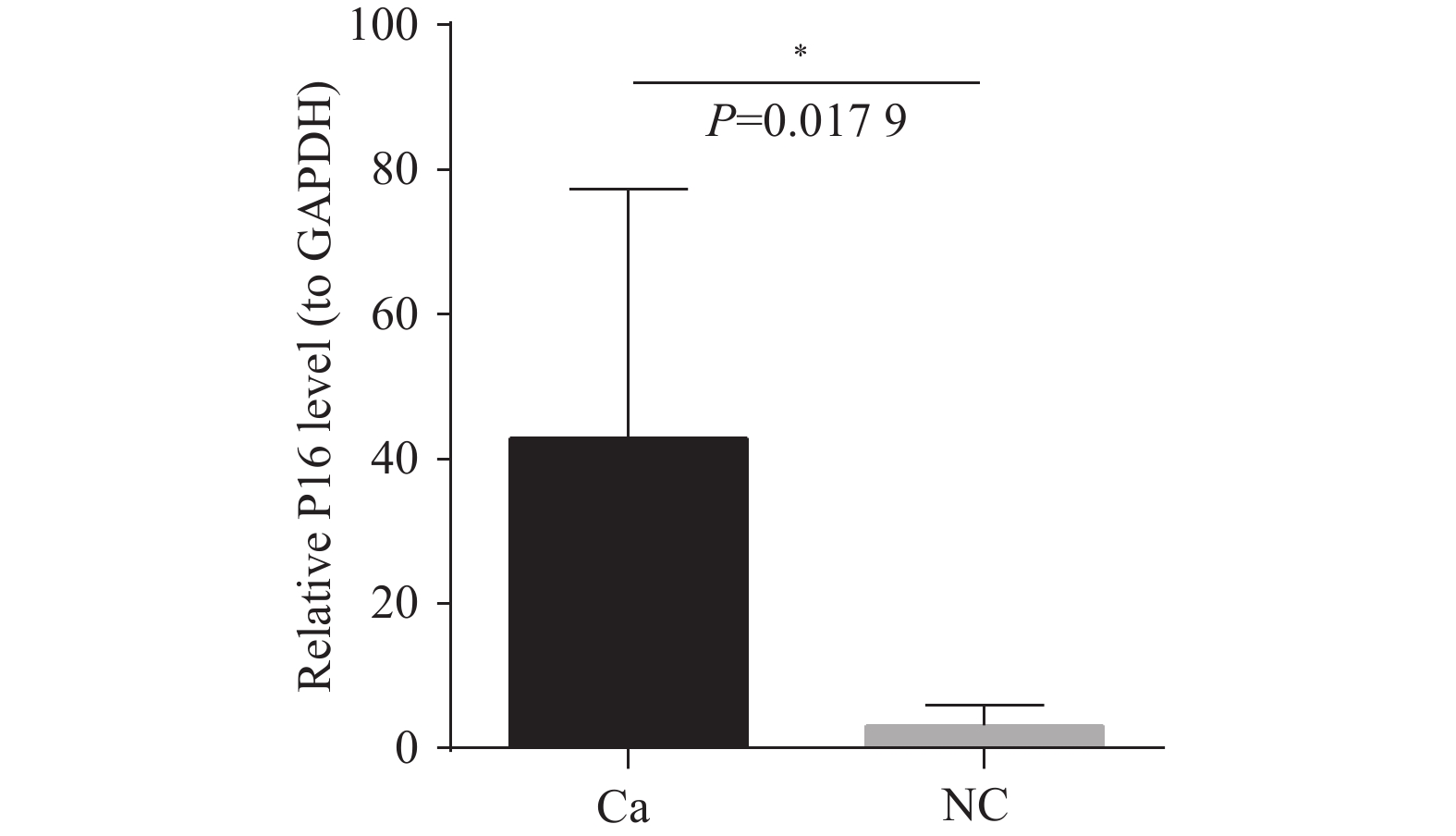
目的 通过临床水平和细胞水平验证p16的表达模式,并进一步探索p16对人胆囊癌(gallbladder cancer,GBC)细胞周期及脂肪酸代谢的影响。 方法 胆囊标本取自2021年1月至2022年12月在昆明医科大学附属第二医院的60例接受胆囊切除的患者。其中胆囊结石伴胆囊炎标本30例,GBC标本30例。采用实时荧光定量反转录PCR(RT-qPCR)检测各GBC样本及胆囊炎样本的p16mRNA的水平、采用免疫组织化学染色检测各GBC样本及胆囊炎样本的p16蛋白表达水平。利用肿瘤细胞的CCK-8检测稳转细胞株的增殖能力、采用流式细胞技术检测肿瘤细胞的凋亡和分裂周期。RT-qPCR检测低表达p16癌细胞中过氧化物酶体相关基因;线粒体氧化相关基因;脂肪酸合成相关基因的表达情况。 结果 p16mRNA及p16蛋白在GBC中的表达显著高于慢性胆囊炎(P < 0.05)。低表达p16之后,肿瘤细胞凋亡率降低,G0/G1期降低,S期升高,细胞分裂加快,差异有统计学意义(P < 0.0001 )。低表达p16之后,GBC中的大部分相关基因显著上调,差异具有统计学意义(P < 0.05)。结论 p16在GBC中显著高表达。p16能够抑制GBC细胞周期的进展。p16可能通过调节脂肪酸代谢的途径影响GBC细胞的发生发展。 Abstract:Objective To verify the expression pattern of p16 at the clinical and cellular levels, and further explore the effects of p16 on the cell cycle and fatty acid metabolism of human gallbladder cancer (GBC) cells. Methods The gallbladder specimens were collected from 60 patients who underwent cholecystectomy at the Second Affiliated Hospital of Kunming Medical University from January 2021 to December 2022. Among them, 30 specimens were from cases of gallstones with cholecystitis, and 30 specimens were from gallbladder cancer (GBC). Real-time fluorescent quantitative reverse transcription PCR (RT-qPCR) was used to detect p16 mRNA levels in GBC samples and cholecystitis samples. Immunohistochemical staining was performed to detect p16 protein expression levels in GBC samples and cholecystitis samples. CCK-8 assay was used to detect the proliferative capacity of stably transfected cell lines. Flow cytometry was employed to detect apoptosis and cell cycle progression in tumor cells. RT-qPCR was used to detect the expression of peroxisome-related genes, mitochondrial oxidation-related genes, and fatty acid synthesis-related genes in cancer cells with low p16 expression. Results The expression of p16 mRNA and p16 protein in GBC was significantly higher than that in chronic cholecystitis (P < 0.05). After p16 downregulation, the apoptosis rate of tumor cells decreased, the proportion of cells in G0/G1 phase decreased, the S phase proportion increased, and cell division accelerated, with statistically significant differences (P < 0.0001 ). After p16 downregulation, most related genes in GBC were significantly upregulated, with statistically significant differences (P < 0.05).Conclusions p16 is significantly highly expressed in GBC. p16 can inhibit the progression of GBC cell cycle. p16 may affect the occurrence and development of GBC cells through regulating the pathway of fatty acid metabolism. -
Key words:
- p16 /
- Gallbladder cancer /
- Cell cycle /
- Fatty acid metabolism /
- Tumor
-
表 1 引物及序列
Table 1. The sequences of the primers
基因 正向引物序列(5′-3′) 反向引物序列(5′-3′) GAPDH CCTTCCGTGTTCCTACCCC GCCCAAGATGCCCTTCAGT CDK4 CGGCCTGTGTCTATGGTCG CAGTCGCCTCAGTAAAGCCA CDK6 TCTGATTACCTGCTCCGCGA CCTCCTCTTCCCTCCTCGAA ACOX1 TTACACACATCCTGGACGGC AATTGAGGCCCACAGGTTCC EHHADH TCAACGCGATCAGGTAACGG TCAACGCGATCAGGTAACGG PPARα CCCTGTCTGCTCTGTGGACT GAGCTCCAAGCTGTGGTGACA CPT1a GACAGCTACGCCAAATCTCTAC GGACATGACGTACTCCCAAAG ACADVL GCGATGACAGCAAAGACGAT GACGGGTATGGGAACACCTG ACACA GACTGGGTAGAGCGATTGATG CTGACAGAGGACTGATGTGATG ACSL1 TTTTGTTAAGTGCTGGTGCC ACCACCACTACCCATGACAG FASN CTGAAGGACCTGTCTAGGTTTG CGGAGTGAATCTGGGTTGAT P16 TTCCTGGACACGCTGGT ACCTTCCGCGGCATCTAT 表 2 干扰靶点
Table 2. Interference target
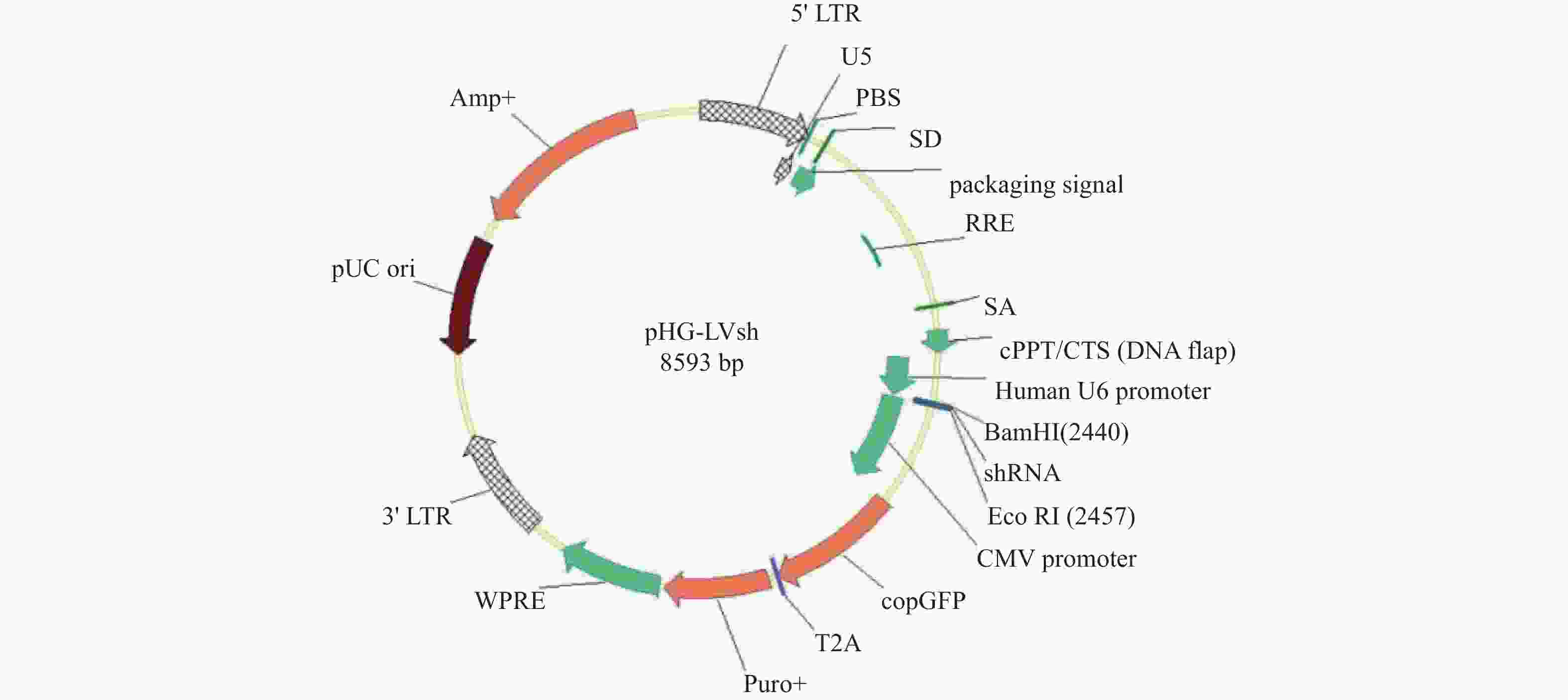
编号 Target序列信息 靶点区域 CDKN2A-sh1 AGTAACCATGCCCGCATAGCT 3′UTR区 CDKN2A-sh2 CCTCGGGAAACTTAGATCATC 3′UTR区 CDKN2A-sh3 CACTACCGTAAATGTCCATTT 3′UTR区 -
[1] Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods[J]. Int J Cancer, 2019, 144(8): 1941-1953. doi: 10.1002/ijc.31937 [2] Hundal R, Shaffer E A. Gallbladder cancer: Epidemiology and outcome[J]. Clin Epidemiol, 2014, 6(1): 99-109. [3] Kamb A, Shattuck-Eidens D, Eeles R, et al. Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus[J]. Nat Genet, 1994, 8(1): 23-26. [4] Barbacid M, Ortega S, Sotillo R, et al. Cell cycle and cancer: Genetic analysis of the role of cyclin-dependent kinases[J]. Cold Spring Harb Symp Quant Biol, 2005, 70: 233-240. doi: 10.1101/sqb.2005.70.005 [5] Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer[J]. Biochim Biophys Acta, 2002, 1602(1): 73-87. [6] Sherr C J, Bertwistle D, DEN Besten W, et al. p53-Dependent and -independent functions of the Arf tumor suppressor[J]. Cold Spring Harb Symp Quant Biol, 2005, 70: 129-137. doi: 10.1101/sqb.2005.70.004 [7] Deleye Y, Cotte A K, Hannou S A, et al. CDKN2A/p16INK4a suppresses hepatic fatty acid oxidation through the AMPKα2-SIRT1-PPARα signaling pathway[J]. J Biol Chem, 2020, 295(50): 17310-17322. doi: 10.1074/jbc.RA120.012543 [8] 高丽丽, 刘照南, 贺亚敏, 等. 胆囊肿瘤临床病理学特征研究进展[J]. 中国实用外科杂志, 2022, 42(8): 947-952+957. doi: 10.19538/j.cjps.issn1005-2208.2022.08.19 [9] 石明亮, 王晓磊, 郭伟, 等. 胆囊癌组织p16INK4a基因甲基化状态和蛋白表达及其与预后的相关性[J]. 社区医学杂志, 2025, 23(9): 292-298. doi: 10.19790/j.cnki.JCM.2025.09.02 [10] 徐燕, 徐小晶, 王远航, 等. DNA倍体联合宫颈脱落细胞p16INK4a对不典型鳞状上皮细胞病变的诊断价值[J]. 安徽医学, 2023, 44(1): 88-92. [11] 李起, 吴予涵, 张瑞, 等. TP53、P16及K-ras在胆囊高级别上皮内瘤变及早期癌中的表达及随机森林预测模型的建立[J]. 西安交通大学学报(医学版), 2021, 42(1): 18-24. doi: 10.7652/jdyxb202101004 [12] Farnaz T, Bhattacharjee P, Ahamad M S U, et al. The prevalence of p16 expression in urothelial bladder cancer in a tertiary care hospital of Chattogram, Bangladesh[J]. Iran J Pathol, 2023, 18(3): 306-311. doi: 10.30699/ijp.2023.1987551.3070 [13] 焦凌玉, 黄立娜, 李晓丽. 膀胱癌组织中ILK、P16表达与临床病理特征和Cyclin D1的关系[J]. 湖北民族大学学报(医学版), 2023, 40(4): 18-20+24. doi: 10.13501/j.cnki.42-1590/r.2023.04.026 [14] 初桂伟, 王利伟, 赵月, 等. nm23-H1和P16蛋白在膀胱癌组织中的表达及其临床意义[J]. 解放军医药杂志, 2018, 30(12): 27-30. doi: 10.3969/j.issn.2095-140X.2018.12.007 [15] Li Y, Zhang T, Zhang H, et al. Clinical significance of P16 gene methylation in lung cancer[J]. Adv Exp Med Biol, 2020, 1255: 133-142. [16] 赵欢, 张萱, 韩志峰, 等. 血浆p16、ANXA-1抗体在非小细胞肺癌患者的表达变化及其作为诊断标志物的探讨[J]. 中国免疫学杂志, 2021, 37(11): 1376-1382. doi: 10.3969/j.issn.1000-484X.2021.11.018 [17] Pezzuto A, D’Ascanio M, Ricci A, et al. Expression and role of p16 and GLUT1 in malignant diseases and lung cancer: A review[J]. Thorac Cancer, 2020, 11(11): 3060-3070. doi: 10.1111/1759-7714.13651 [18] Xue L, Wang X, Yang Y, et al. Segnet network algorithm-based ultrasound images in the diagnosis of gallbladder stones complicated with gallbladder carcinoma and the relationship between P16 expression with gallbladder carcinoma[J]. J Healthc Eng, 2021, 2021: 2819986. [19] Choi H J, Yun S S, Kim H J, et al. Expression of p16 protein in gallbladder carcinoma and its precancerous conditions[J]. Hepatogastroenterology, 2010, 57(97): 18-21. [20] Nepal C, Zhu B, O’Rourke C J, et al. Integrative molecular characterisation of gallbladder cancer reveals micro-environment-associated subtypes[J]. J Hepatol, 2021, 74(5): 1132-1144. doi: 10.1016/j.jhep.2020.11.033 [21] Norbury C, Nurse P. Animal cell cycles and their control[J]. Annu Rev Biochem, 1992, 61: 441-470. doi: 10.1146/annurev.bi.61.070192.002301 [22] Wang X Y, Wang Y G, Wang Y F. Ginsenoside Rb1, Rg1 and three extracts of traditional Chinese medicine attenuate ultraviolet B-induced G1 growth arrest in HaCaT cells and dermal fibroblasts involve down-regulating the expression of p16, p21 and p53[J]. Photodermatol Photoimmunol Photomed, 2011, 27(4): 203-212. doi: 10.1111/j.1600-0781.2011.00601.x [23] 张君薇, 王国秋. P16蛋白在胆囊癌组织中的表达[J]. 锦州医学院学报, 2005, 26(1): 24-25. doi: 10.3969/j.issn.1671-038X.2005.04.017 [24] 马红兵, 王作仁, 狄政莉, 等. 胆囊癌组织p16和cyclin D1表达[J]. 第四军医大学学报, 2003, 24(18): 1665-1667. doi: 10.3321/j.issn:1000-2790.2003.18.011 [25] 周文策, 李玉民, 张煦, 等. P16、CD44v3在胆囊癌中的表达及临床意义[J]. 兰州大学医学报(医学版), 2005, 31(4): 1-4. doi: 10.3969/j.issn.1000-2812.2005.04.001 [26] Yuan H X, Wang C, Tang C Y, et al. Differential diagnosis of gallbladder neoplastic polyps and cholesterol polyps with radiomics of dual modal ultrasound: A pilot study[J]. BMC Med Imaging, 2023, 23(1): 26-35. doi: 10.1186/s12880-023-00982-y [27] Gross B, Pawlak M, Lefebvre P, et al. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD[J]. Nat Rev Endocrinol, 2017, 13(1): 36-49. doi: 10.1038/nrendo.2016.135 [28] Tan Y, Wang M, Yang K, et al. PPAR-α modulators as current and potential cancer treatments[J]. Front Oncol, 2021, 11: 599995. doi: 10.3389/fonc.2021.599995 [29] Zhao Z D, Zan L S, Li A N, et al. Characterization of the promoter region of the bovine long-chain acyl-CoA synthetase 1 gene: Roles of E2F1, Sp1, KLF15, and E2F4[J]. Sci Rep, 2016, 6: 19661. doi: 10.1038/srep19661 [30] Zhang Q, Li N, Deng L, et al. ACSL1-induced ferroptosis and platinum resistance in ovarian cancer by increasing FSP1 N-myristylation and stability[J]. Cell Death Discov, 2023, 9(1): 83. doi: 10.1038/s41420-023-01385-2 -





 下载:
下载: