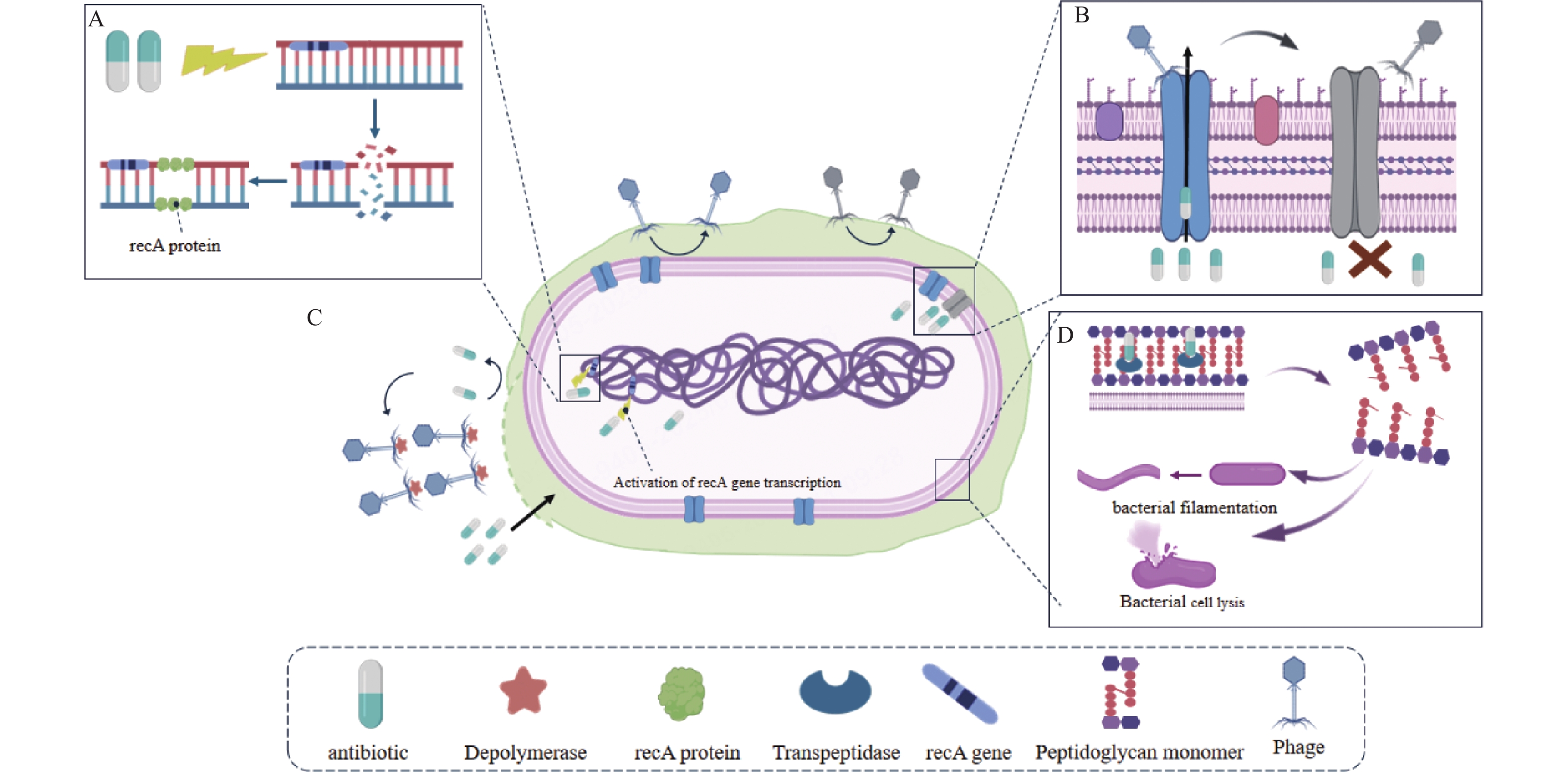
-
摘要: 抗生素滥用导致耐药菌迅速增加,细菌感染难以有效控制,对人类健康构成严重威胁。随着抗生素耐药性问题的日益加剧和新抗生素研发滞后,寻找更有效的治疗策略成为全球公共卫生领域的迫切需求。噬菌体作为潜在治疗方案,近年来受到广泛关注,但噬菌体存在宿主范围狭窄、溶源性现象等局限性。噬菌体-抗生素协同作用通过增强噬菌体裂解活性,减少抗生素剂量,显著降低耐药风险,展现出卓越的抗菌效果。本文综述了噬菌体-抗生素协同作用的机制及研究进展,以期进一步推动噬菌体与抗生素联合疗法的研究与应用,为应对抗生素耐药性问题提供新的解决方案。Abstract: The overuse of antibiotics has led to a rapid increase of antibiotic-resistant bacteria, making bacterial infections difficult to control effectively and posing a serious threat to human health. As antibiotic resistance problems become increasingly severe and the development of new antibiotics lags, finding more effective treatment strategies has become an urgent need in global public health. Bacteriophages, as a potential therapeutic option, have attracted increasing attention in recent years; however, bacteriophages have limitations such as a narrow host range and lysogeny. Synergistic effects between bacteriophages and antibiotics enhance bacteriophage lytic activity, reduce antibiotic dosage, significantly lower the risk of resistance development, and demonstrate superior antimicrobial efficacy. This review summarizes the mechanisms and research progress of bacteriophage-antibiotic synergy, aiming to further advance research and clinical application of combined bacteriophage-antibiotic therapy and provide new solutions to address the problem of antibiotic resistance.
-
Key words:
- Bacteriophage /
- Antibiotic /
- Combination therapy /
- Resistance /
- Synergistic effects
-
表 1 临床实践中噬菌体-抗生素的联合治疗
Table 1. Phages and antibiotics in clinical practice
Infection Case presentation Phage Antibiotic Clinical outcome Reference 1 Multidrug resistant A. baumannii and K. pneumoniae infection A 42-year-old patient with a trauma-related left tibial infection with drug resistant A. baumannii and K. pneumoniae ϕAbKT21phi3 and ϕKpKT21phi1 Meropenem and colistin Rapid tissue healing and positive culture eradication. The patient’s leg did not have to be amputated and he is undergoing rehabilitation [34] 2 Pandrug-resistant K. pneumoniae A 30-year-old patient with a fracture-associated pandrug-resistant K. pneumoniae infection. phage M1 Meropenem and colistin followed by ceftazidime/avibactam The patient regained the ability to walk and resume activities,with no recurrence of Klebsiella pneumoniae infection. [35] 3 Pseudomonas aeruginosa infection A 75-year-old woman with arteriosclerosis and comorbidities developed Pseudomonas aeruginosa infection after femoropopliteal bypass surgery in the right inguinal area. fNenPA2p2、fNenPA2p4 and fGstaPae02 Meropenem Infection markers normalized. PET-CT showed clinical improvement. No further Pseudomonas aeruginosa septicemia occurred during the 10-month follow-up. [36] 4 Methicillin-Susceptible Staphylococcus Aureus infection A 29-year-old woman with type-1 neurofibromatosis developed an MSSA surgical site infection with a skin fistula one month after ceramic cranioplasty,leading to septic shock and free flap necrosis. two different
bacteriophagesDalbavancin Within two weeks of combination therapy,purulent discharge gradually decreased to no exudate. At 8 months after treatment,the patient remains healthy with no infection recurrence. [37] 5 Multidrug resistant Acinetobacter baumannii (MDR-A) respiratory infection A 52-year-old critically ill patient with MDR A. baumannii respiratory infection A.baumannii phage AbW4878Ø1 Tigecycline、Trimethoprim/sulfametoxazole Successfully treated with antibiotics and intravenous and nebulized PT. [38] 6 Multidrug resistant (MDR) P. aeruginosa pneumonia infection A 26-year-old cystic fibrosis (CF) patient awaiting lung transplantation with multidrug resistant (MDR) P. aeruginosa pneumonia AB-PA01 Iprofloxacin、piperacillin–tazobactam and doripenem No worsening or recurrence of pneumonia and cystic fibrosis within 100 days after phage therapy. [39] -
[1] Marshall B M, Levy S B. Food animals and antimicrobials: Impacts on human health[J]. Clin Microbiol Rev, 2011, 24(4): 718-733. doi: 10.1128/CMR.00002-11 [2] Reygaert W C. An overview of the antimicrobial resistance mechanisms of bacteria[J]. AIMS Microbiol, 2018, 4(3): 482-501. doi: 10.3934/microbiol.2018.3.482 [3] Gordillo Altamirano F L, Barr J J. Phage therapy in the postantibiotic era[J]. Clin Microbiol Rev, 2019, 32(2): e00066-18. [4] Safir M C, Bhavnani S M, Slover C M, et al. Antibacterial drug development: A new approach is needed for the field to survive and thrive[J]. Antibiotics, 2020, 9(7): 412. doi: 10.3390/antibiotics9070412 [5] Pal N, Sharma P, Kumawat M, et al. Phage therapy: An alternative treatment modality for MDR bacterial infections[J]. Infect Dis, 2024, 56(10): 785-817. doi: 10.1080/23744235.2024.2379492 [6] Kalia V C, Patel S K S, Gong C, et al. Re-emergence of bacteriophages and their products as antibacterial agents: An overview[J]. Int J Mol Sci, 2025, 26(4): 1755. doi: 10.3390/ijms26041755 [7] D'Accolti M, Soffritti I, Mazzacane S, et al. Bacteriophages as a Potential 360- D’Accolti M, Soffritti I, Mazzacane S, et al. Bacteriophages as a potential 360-degree pathogen control strategy[J]. Microorganisms, 2021, 9(2): 261. doi: 10.3390/microorganisms9020261 [8] Diallo K, Dublanchet A. Benefits of combined phage-antibiotic therapy for the control of antibiotic-resistant bacteria: A literature review[J]. Antibiotics, 2022, 11(7): 839. doi: 10.3390/antibiotics11070839 [9] Li X, He Y, Wang Z, et al. A combination therapy of Phages and Antibiotics: Two is better than one[J]. Int J Biol Sci, 2021, 17(13): 3573-3582. doi: 10.7150/ijbs.60551 [10] Łusiak-Szelachowska M, Międzybrodzki R, Drulis-Kawa Z, et al. Bacteriophages and antibiotic interactions in clinical practice: What we have learned so far[J]. J Biomed Sci, 2022, 29(1): 23. doi: 10.1186/s12929-022-00806-1 [11] North O I, Brown E D. Phage-antibiotic combinations: A promising approach to constrain resistance evolution in bacteria[J]. Ann N Y Acad Sci, 2021, 1496(1): 23-34. doi: 10.1111/nyas.14533 [12] Chan B K, Sistrom M, Wertz J E, et al. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa[J]. Sci Rep, 2016, 6: 26717. doi: 10.1038/srep26717 [13] Liu N, Lewis C, Zheng W, et al. Phage cocktail therapy: Multiple ways to suppress pathogenicity[J]. Trends Plant Sci, 2020, 25(4): 315-317. doi: 10.1016/j.tplants.2020.01.013 [14] Kraus S, Fletcher M L, Łapińska U, et al. Phage-induced efflux down-regulation boosts antibiotic efficacy[J]. PLoS Pathog, 2024, 20(6): e1012361. doi: 10.1371/journal.ppat.1012361 [15] Dickey J, Perrot V. Adjunct phage treatment enhances the effectiveness of low antibiotic concentration against Staphylococcus aureus biofilms in vitro[J]. PLoS One, 2019, 14(1): e0209390. doi: 10.1371/journal.pone.0209390 [16] Łusiak-Szelachowska M, Weber-Dąbrowska B, Górski A. Bacteriophages and lysins in biofilm control[J]. Virol Sin, 2020, 35(2): 125-133. doi: 10.1007/s12250-019-00192-3 [17] Bulssico J, PapukashvilI I, Espinosa L, et al. Phage-antibiotic synergy: Cell filamentation is a key driver of successful phage predation[J]. PLoS Pathog, 2023, 19(9): e1011602. doi: 10.1371/journal.ppat.1011602 [18] Comeau A M, Tétart F, Trojet S N, et al. Phage-Antibiotic Synergy (PAS): Beta-lactam and quinolone antibiotics stimulate virulent phage growth[J]. PLoS One, 2007, 2(8): e799. doi: 10.1371/journal.pone.0000799 [19] Supina B S I, Dennis J J. The current landscape of phage-antibiotic synergistic (PAS) interactions[J]. Antibiotics, 2025, 14(6): 545. doi: 10.3390/antibiotics14060545 [20] Nanda A M, Heyer A, Krämer C, et al. Analysis of SOS-induced spontaneous prophage induction in Corynebacterium glutamicum at the single-cell level[J]. J Bacteriol, 2014, 196(1): 180-188. doi: 10.1128/JB.01018-13 [21] Morris T C, Reyneke B, Khan S, et al. Phage-antibiotic synergy to combat multidrug resistant strains of Gram-negative ESKAPE pathogens[J]. Sci Rep, 2025, 15(1): 17235. doi: 10.1038/s41598-025-01489-y [22] Fothergill J L, Mowat E, Walshaw M J, et al. Effect of antibiotic treatment on bacteriophage production by a cystic fibrosis epidemic strain of Pseudomonas aeruginosa[J]. Antimicrob Agents Chemother, 2011, 55(1): 426-428. doi: 10.1128/AAC.01257-10 [23] Goerke C, Köller J, Wolz C. Ciprofloxacin and trimethoprim cause phage induction and virulence modulation in Staphylococcus aureus[J]. Antimicrob Agents Chemother, 2006, 50(1): 171-177. doi: 10.1128/AAC.50.1.171-177.2006 [24] Kim M, Jo Y, Hwang Y J, et al. Phage-antibiotic synergy via delayed lysis[J]. Appl Environ Microbiol, 2018, 84(22): e02085-18. [25] Ghatbale P, Sah G P, Dunham S, et al. In vitro resensitization of multidrug-resistant clinical isolates of Enterococcus faecium and E. faecalis through phage-antibiotic synergy[J]. Antimicrobial Agents and Chemotherapy, 2025, 69(2): e00740-24. [26] 曾堃. 耐药粪肠球菌致根尖周炎的噬菌体-抗生素联合治疗研究[D]. 昆明: 昆明理工大学, 2024. [27] Orozco-Ochoa A K, González-Gómez J P, Quiñones B, et al. Bacteriophage Indie resensitizes multidrug-resistant Acinetobacter baumannii to antibiotics in vitro[J]. Sci Rep, 2025, 15(1): 11578. doi: 10.1038/s41598-025-96669-1 [28] Fatima R, Hynes A P. Temperate phage-antibiotic synergy is widespread-extending to Pseudomonas-but varies by phage, host strain, and antibiotic pairing[J]. mBio, 2025, 16(2): e0255924. doi: 10.1128/mbio.02559-24 [29] Kumaran D, Taha M, Yi Q, et al. Does treatment order matter investigating the ability of bacteriophage to augment antibiotic activity against Staphylococcus aureus biofilms[J]. Front Microbiol, 2018, 9: 127. doi: 10.3389/fmicb.2018.00127 [30] Alharbi M G, Al-Hindi R R, Alotibi I A, et al. Evaluation of phage-antibiotic combinations in the treatment of extended-spectrum β-lactamase-producing Salmonella enteritidis strain PT1[J]. Heliyon, 2023, 9(1): e13077. doi: 10.1016/j.heliyon.2023.e13077 [31] Yehia F A A, Yahya G, Elsayed E M, et al. From isolation to application: Utilising phage-antibiotic synergy in murine bacteremia model to combat multidrug-resistant Enterococcus faecalis[J]. Microb Biotechnol, 2025, 18(1): e70075. doi: 10.1111/1751-7915.70075 [32] El-Telbany M, El-Didamony G, Askora A, et al. Bacteriophages to control multi-drug resistant enterococcus faecalis infection of dental root canals[J]. Microorganisms, 2021, 9(3): 517-530. [33] Zhao M, Li H, Gan D, et al. Antibacterial effect of phage cocktails and phage-antibiotic synergy against pathogenic Klebsiella pneumoniae[J]. mSystems, 2024, 9(9): e0060724. doi: 10.1128/msystems.00607-24 [34] Nir-Paz R, Gelman D, Khouri A, et al. Successful treatment of antibiotic-resistant, poly-microbial bone infection with bacteriophages and antibiotics combination[J]. Clin Infect Dis, 2019, 69(11): 2015-2018. [35] Eskenazi A, Lood C, Wubbolts J, et al. Combination of pre-adapted bacteriophage therapy and antibiotics for treatment of fracture-related infection due to pandrug-resistant Klebsiella pneumoniae[J]. Nat Commun, 2022, 13(1): 302. doi: 10.1038/s41467-021-27656-z [36] Otava U E, Tervo L, Havela R, et al. Phage-antibiotic combination therapy against recurrent Pseudomonas Septicaemia in a patient with an arterial stent[J]. Antibiotics, 2024, 13(10): 916. doi: 10.3390/antibiotics13100916 [37] Bleibtreu A, Fevre C, Robert J, et al. Combining bacteriophages and dalbavancin for salvage therapy of complex Staphylococcus aureus extradural empyema[J]. Med Mal Infect, 2020, 50(5): 458-459. doi: 10.1016/j.medmal.2020.02.004 [38] Rao S, Betancourt-Garcia M, Kare-Opaneye Y O, et al. Critically ill patient with multidrug-resistant Acinetobacter baumannii respiratory infection successfully treated with intravenous and nebulized bacteriophage therapy[J]. Antimicrob Agents Chemother, 2022, 66(1): e00824-21. [39] Law N, Logan C, Yung G, et al. Successful adjunctive use of bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa infection in a cystic fibrosis patient[J]. Infection, 2019, 47(4): 665-668. doi: 10.1007/s15010-019-01319-0 [40] Rao G G, Vallé Q, Mahadevan R, et al. Crossing the chasm: How to approach translational pharmacokinetic-pharmacodynamic modeling of phage dosing[J]. Clin Pharmacol Ther, 2025, 117(1): 94-105. doi: 10.1002/cpt.3426 [41] Kim M K, Chen Q, Echterhof A, et al. A blueprint for broadly effective bacteriophage-antibiotic cocktails against bacterial infections[J]. Nat Commun, 2024, 15(1): 9987. doi: 10.1038/s41467-024-53994-9 [42] Dedrick R M, Guerrero-Bustamante C A, Garlena R A, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus[J]. Nat Med, 2019, 25(5): 730-733. doi: 10.1038/s41591-019-0437-z [43] Hibstu Z, Belew H, Akelew Y, et al. Phage therapy: A different approach to fight bacterial infections[J]. Biologics, 2022, 16: 173-186. [44] Rahimian M, Jafari-Gharabaghlou D, Mohammadi E, et al. A new insight into phage combination therapeutic approaches against drug-resistant mixed bacterial infections[J]. Phage, 2024, 5(4): 203-222. doi: 10.1089/phage.2024.0011 -





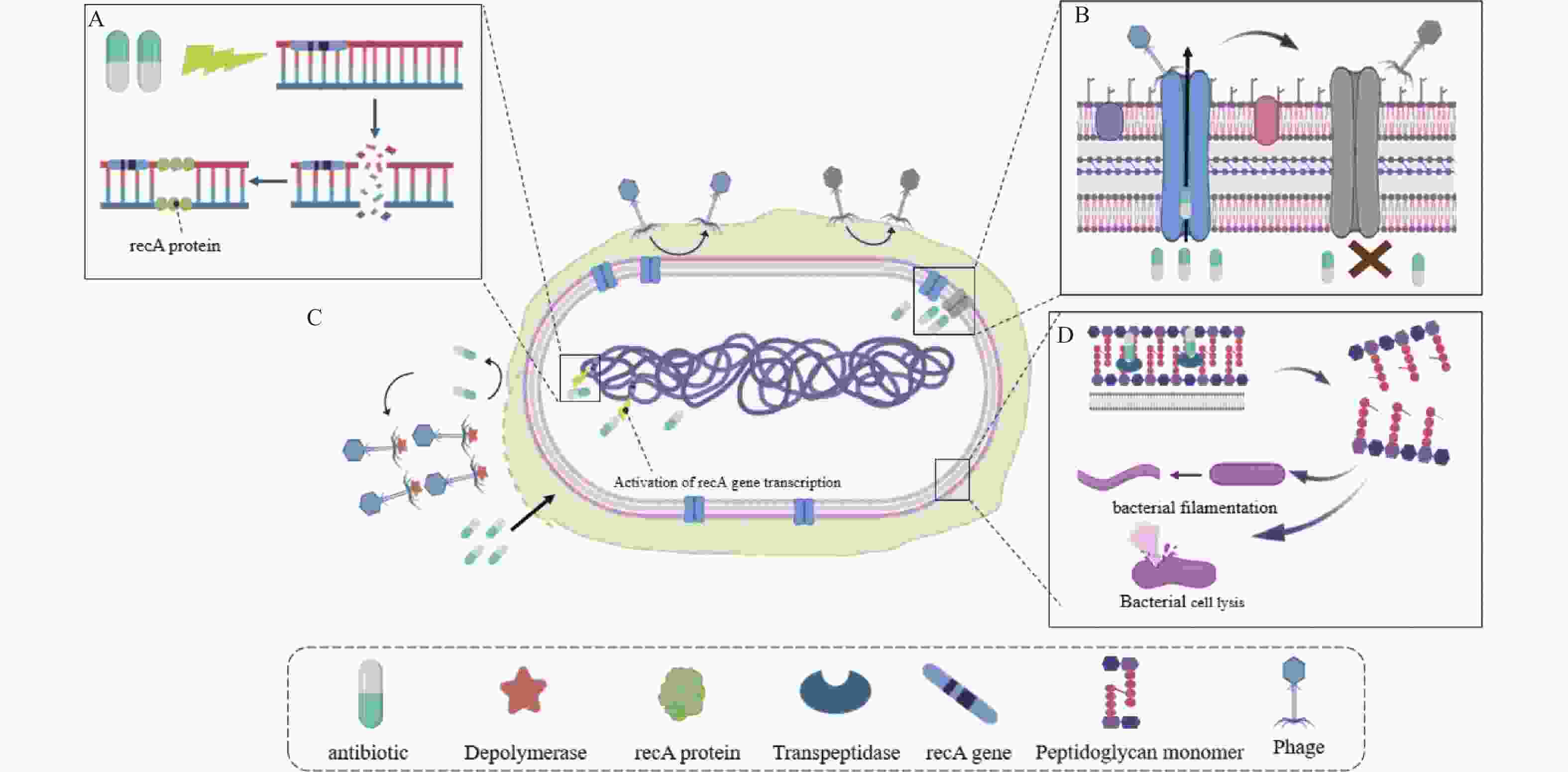
 下载:
下载: