miR-490-3p Suppresses EMT of SW1990 Pancreatic Cancer Cells
-
摘要:
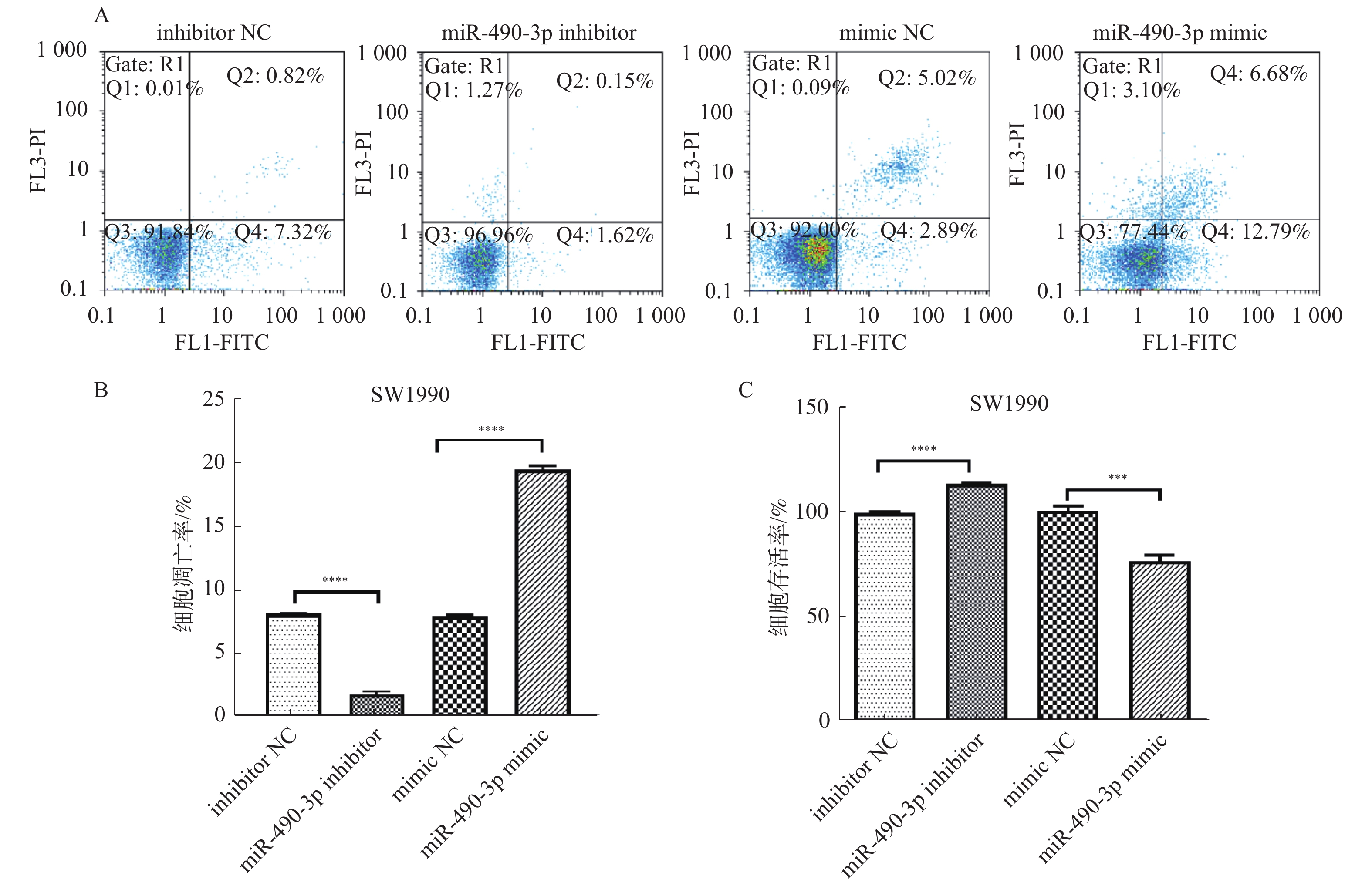
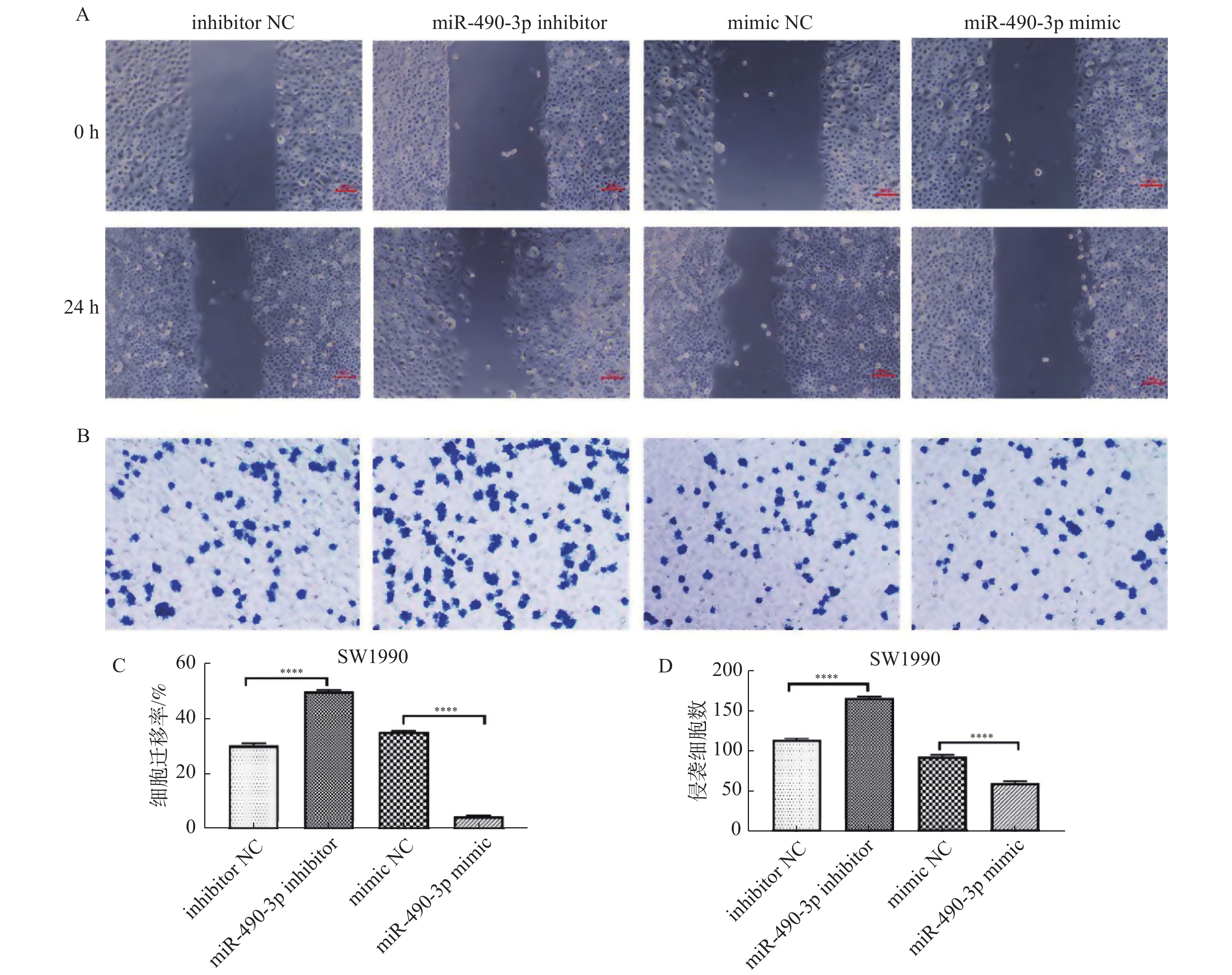
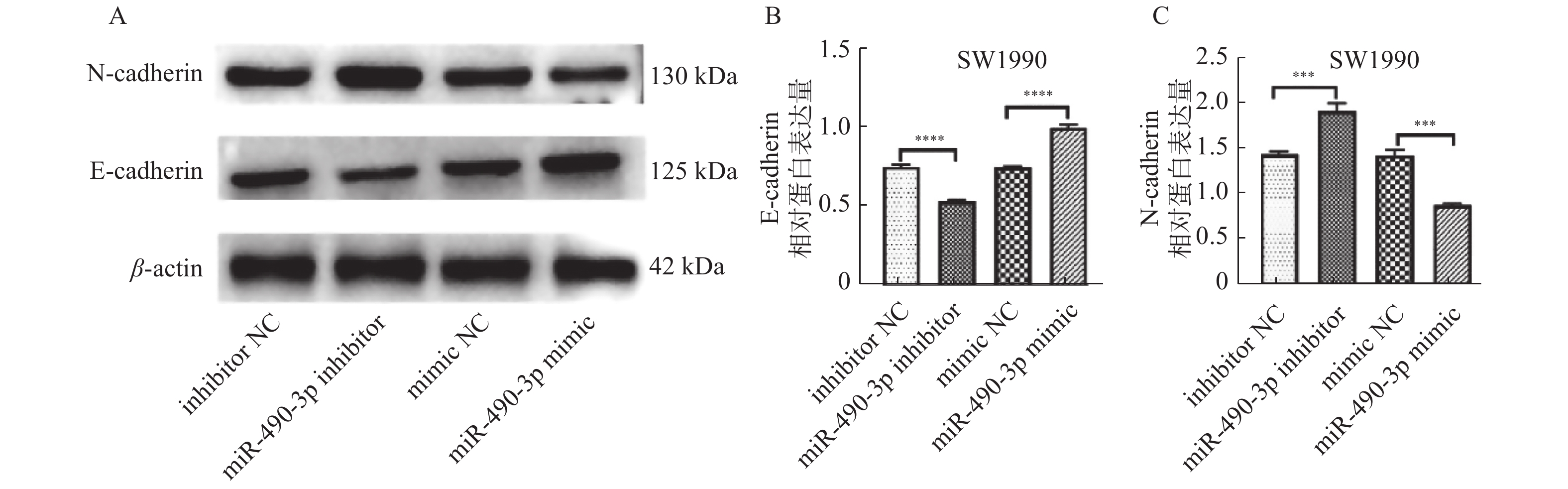
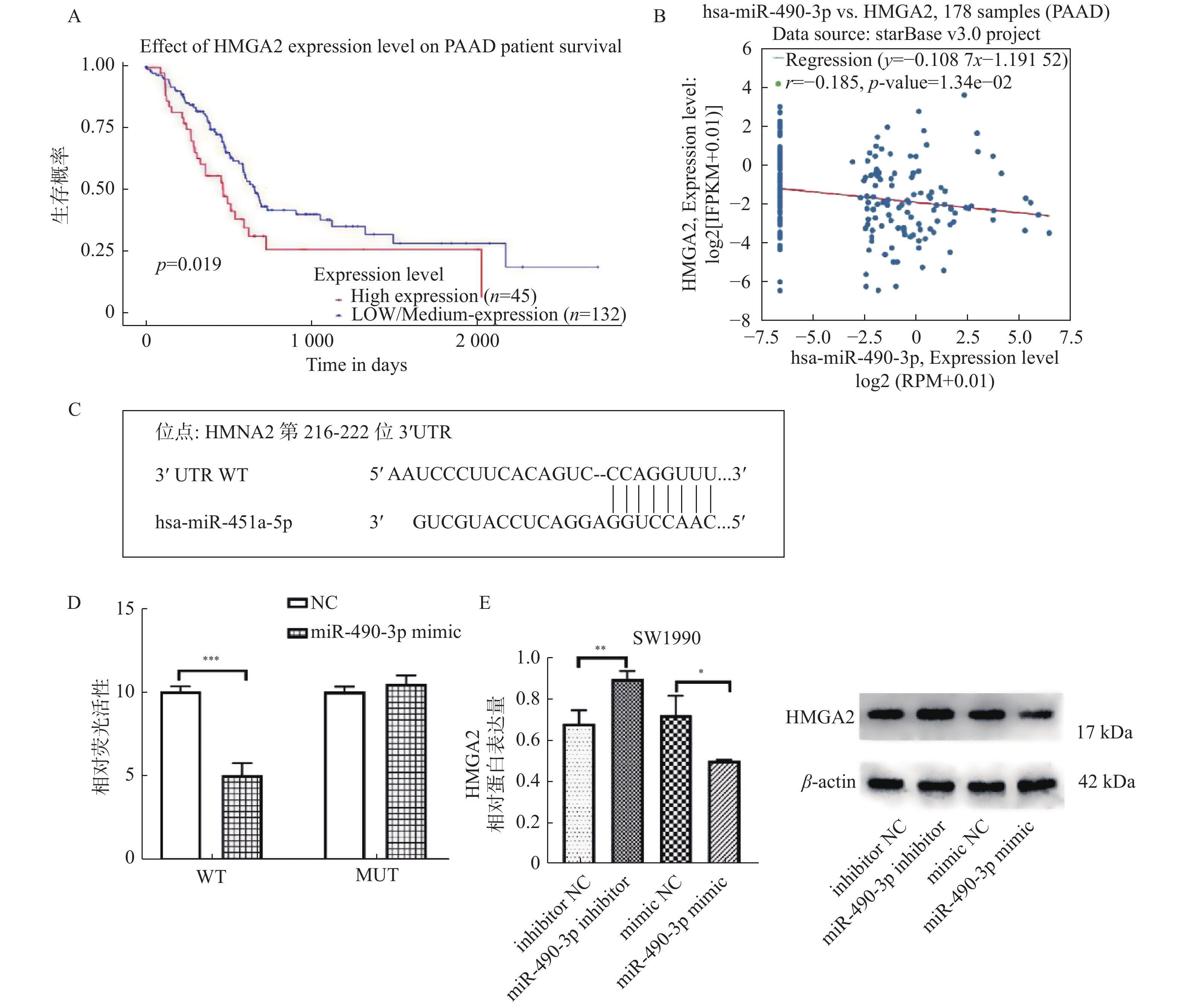
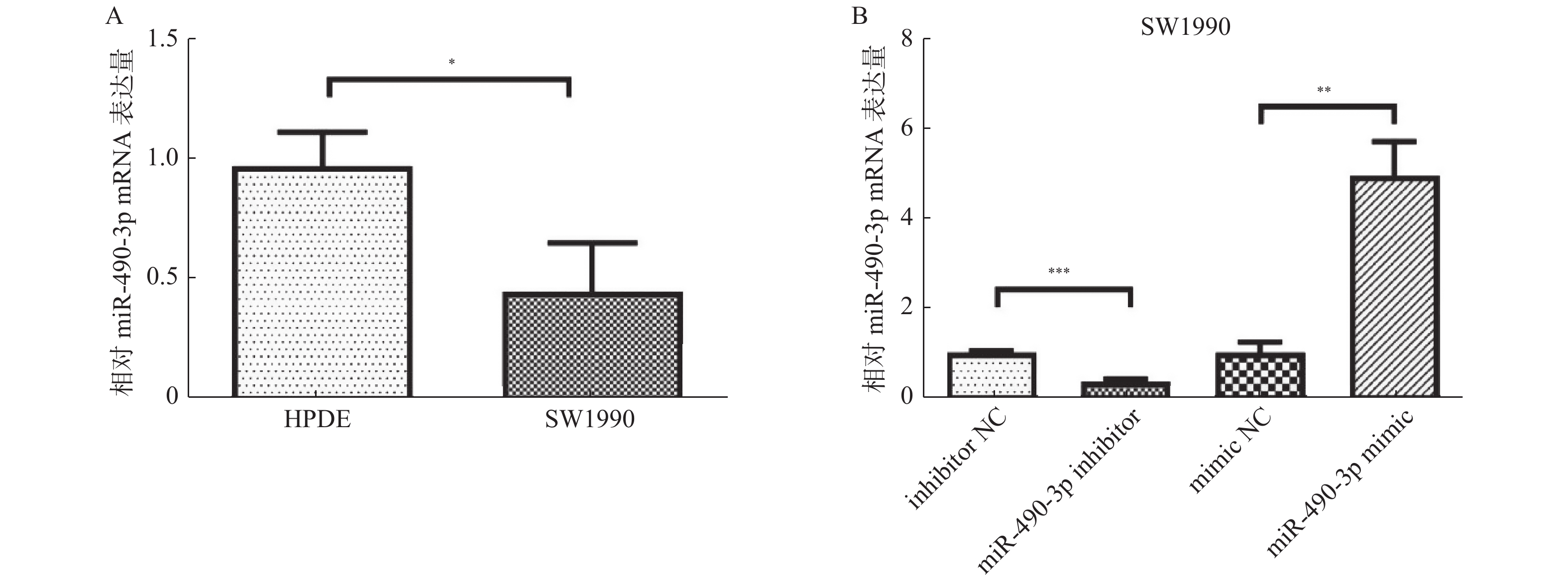
目的 研究miR-490-3p在分子水平上通过HMGA2蛋白对SW1990细胞上皮间充质转化的影响。 方法 通过实时荧光定量PCR法(RT-qPCR)检测正常胰腺导管上皮细胞HPDE和人胰腺癌细胞SW1990中miR-490-3p的表达。通过转染质粒[miR-490-3p阻遏物(inhibitor)和miR-490-3p模拟物(mimic)以及各自的阴性对照质粒]调控miR-490-3p在SW1990细胞中的表达并通过RT-qPCR法验证转染效率。转染48 h后,通过CCK8检测、划痕法、Transwell小室检测,流式细胞仪检测等分别检测细胞增殖、凋亡、迁移和侵袭等恶性生物学特征的影响。通过Western blot检测细胞中上皮间充质转化相关蛋白E-cadherin和N-cadherin的蛋白表达水平。同时,用数据库预测miR-490-3p及其靶基因HMGA2的靶向关系,并通过双荧光素酶报告基因实验进行验证,通过Western blot检测细胞中HMGA2的蛋白表达水平。 结果 (1)与HPDE正常细胞相比,SW1990癌细胞中miR-490-3p表达水平明显降低(P = 0.215);(2)miR-490-3p 上调后,SW1990细胞的凋亡率显著高于对照组(P < 0.0001),而细胞增殖、迁移和侵袭能力显著低于对照组(P < 0.0001)。miR-490-3p 下调后,SW1990细胞的凋亡率显著低于对照组(P < 0.0001),而细胞增殖、迁移和侵袭能力显著高于对照组(P < 0.0001);(3)miR-490-3p 上调后,SW1990细胞中的N-cadherin表达量显著高于对照组(P < 0.0001),而E-cadherin的表达水平显著低于对照组(P < 0.0001);miR-490-3p 下调后,SW1990细胞中的N-cadherin表达量显著低于对照组(P < 0.0001),而E-cadherin的表达水平显著高于对照组(P < 0.0001);(4)HMGA2是miR-490-3p的靶向基因。 结论 miR-490-3p可通过靶向HMGA2抑制SW1990胰腺癌细胞EMT,从而影响胰腺癌的的发生发展进程,揭示HMGA2和miR-490-3p可能为胰腺癌诊断和治疗的靶点。 -
关键词:
- 胰腺癌 /
- 上皮间充质转化 /
- miR-490-3p /
- HMGA2
Abstract:Objective To elucidate the role of miR-490-3p in regulating HMGA2 expression and affecting epithelial-mesenchymal transition (EMT) of SW1990 cells. Methods miR-490-3p expression was measured in HPDE and SW1990 cell lines by Real-time PCR. SW1990 cell lines were cultured in vitro and divided into four groups, including inhibitor-Negative control (inhibitor-NC), miR-490-3p inhibitor, mimic- Negative control (mimic-NC) and miR-490-3p mimic group, and the transfection efficiency was verified by RT-qPCR. After 48 h of transfection, CCK8 assay, wound healing assay, Transwell invasion assay andflow cytometric were used to detect the effects of malignant biological characteristics such as proliferation, migration, invasion and apoptosis of SW1990 cell linerespectively. Western blot was used to detect the expression of E-cadherin and N-cadherin. Meanwhile, database predicted the target genes of miR-490-3p, and dual luciferase assay confirmed the targeted relationship between miR-490-3p and HMGA2, western blot was used to detect the expression of HMGA2. Results 1) The expression of miR-490-3p was downregulated in SW1990 cells (P = 0.215). 2) Overexpression of miR-490-3p significantly inhibited the proliferation (P < 0.0001), migration (P < 0.0001) and invasion (P < 0.0001) of SW1990 cell line and prompted apoptosis (P < 0.0001); In contrast, downregulation of miR-490-3p significantly promoted the proliferation (P < 0.0001), migration (P < 0.0001) and invasion (P < 0.0001) of SW1990 cell line and inhibited apoptosis (P < 0.0001). 3) Upregulation of miR-490-3p significantly decreased the expression of N-cadherin (P < 0.0001) and increased E-cadherin (P < 0.0001) expression in SW1990 cell line. In contrast, downregulation of miR-490-3p significantly upregulated (P < 0.0001) the expression of N-cadherin, decreased E-cadherin (P < 0.0001) expression. 4) HMGA2 was a target gene of miR-490-3p. Conclusion These findings indicate that miR-490-3p acts as a tumor suppressor during the process of EMT through targeting HMGA2, suggesting miR-490-3p is a potential new diagnostic and therapeutic target for the treatment of pancreatic cancer. -
Key words:
- Pancreatic cancer /
- EMT /
- miR-490-3p /
- HMGA2
-
烟草危害是当今世界最严重的公共卫生问题之一,我国仍是世界上最大的烟草生产国和消费国,全国烟民数量超过3亿,7.4亿人受二手烟危害,每年因吸烟导致疾病的死亡人数超过100万[1]。吸烟对健康的危害已得到大量科学研究的证实,与包括肺癌、食管癌、胃癌、胰腺癌、肾癌、膀胱癌、宫颈癌、直肠癌等在内的多种癌症密切相关[2]。任何人在任何年龄戒烟均可获益,且戒烟越早、持续时间越长,健康获益越大。
目前我国临床已批准使用的戒烟药物包括尼古丁贴片、尼古丁咀嚼胶(非处方药)、盐酸安非他酮缓释片(处方药)、伐尼克兰(处方药),这些药物单独应用于戒烟治疗的疗效已获得肯定,但并非所有烟草依赖患者都能通过单药疗法达到戒烟效果,尤其重度烟草依赖患者常伴有明显戒断症状,导致复吸[3]。为提高戒烟率,探究已被证实单药治疗有效的药物进行组合,寻找提高戒烟率和减少戒断症状的疗法组合[4]。我国戒烟指南中指出已被证实有效的药物组合包括:长疗程尼古丁贴片治疗(> 14周)+其他尼古丁替代疗法(nicotine replacement therapy,NRT)类药物(如咀嚼胶);尼古丁贴片+盐酸安非他酮缓释片[5]。 但是,关于伐尼克兰联合尼古丁贴剂的研究非常有限。现对其进行综述,为该联合疗法在戒烟治疗中提供依据。
1. 尼古丁依赖的发生机制
烟草中的主要成瘾物质是尼古丁,尼古丁通过作用于外周及中枢神经系统内的烟碱受体,影响多种神经递质释放量改变,导致一系列生理表现发生而形成依赖。其中最主要机制是以中脑边缘多巴胺回路为基础的尼古丁奖赏效应。普通燃烧卷烟中尼古丁以烟雾颗粒的形式进入肺部,迅速被肺循环吸收,随血液循环通过血脑屏障扩散到脑组织,作用于其中的烟碱受体。研究显示,人脑中最丰富的受体亚型是α4β2、α3β4和α7(同型)。而α4β2受体亚型在人脑中占主导地位,目前认为是介导尼古丁依赖发生的主要受体[6]。尼古丁与受体结合后促使兴奋性神经递质多巴胺大量释放而使吸烟者产生“愉悦感”,当脑内尼古丁水平下降时,相关奖赏感受减弱或消失,并出现戒断症状。此外,长期反复尼古丁摄入可致脑内烟碱受体结合位点数增加,产生受体脱敏效应,使吸烟者不断强化吸烟行为,导致依赖及耐受[7]。
尼古丁替代疗法通过部分替代从吸烟中获得的尼古丁,缓解吸烟渴求及戒断症状,辅助戒烟。尼古丁替代疗法包括尼古丁咀嚼胶、透皮贴剂、鼻喷剂、吸入器、含片等。不同剂型的NRT类药物在戒烟疗效方面无显著差别,其中尼古丁贴剂因其较高的依从性通常作为首选。咀嚼胶、鼻喷剂、吸入器、含片等产品较贴剂起效快,但作用时间短,需要频繁给药而影响药物依从性。且当吸烟者为缓解戒断症状而滥用这些速效替代产品时,可显著增加血中尼古丁浓度达到与吸烟时相同水平,影响戒烟效果[8]。就尼古丁咀嚼胶而言,使用者正确的咀嚼技巧是药物起效及减少不良反应发生的必要条件。相比其他尼古丁替代疗法产品,贴剂释放尼古丁的速率更稳定,作用更温和。因此本文选择尼古丁贴剂作为替代疗法联合伐尼克兰疗效进行综述。
2. 伐尼克兰联合尼古丁替代疗法可能的作用机制
伐尼克兰是一种高选择性的α4β2 nAchR(nicotinic acetylcholine receptor,nAchR)部分激动剂。α4β2 nAchR广泛分布于中脑边缘多巴胺(dopamine,DA)细胞上,卷烟中的尼古丁释放后与α4β2 nAchR结合,促DA释放并作用于伏隔核(nucleus accumbens,NAc),使吸烟者产生“愉悦感”和其他奖赏感受。伐尼克兰通过对α4β2和α6β2受体亚型激动及拮抗的双重调节作用,阻断尼古丁诱导的DA释放,以达到减少戒断症状及提高戒烟率的效果[9]。
同样作用于α4β2 nAchR的尼古丁贴剂主要通过缓慢释放其有效成分,维持相对较低的血浆尼古丁水平,缓解焦虑和戒断症状。二者联合使用理论上因伐尼克兰的部分拮抗作用而难以获益,但既往对此所进行的临床试验结论不一[10−14],对该联合疗法疗效的探索主要基于以下可能作用机制:(1)伐尼克兰不能使α4β2受体完全饱和,为尼古丁替代疗法留下作用空间。基于此假设,重度烟草依赖人群理论上更能从中获益,但缺少相关临床试验研究。Lotfipour等[15]对伐尼克在人脑中的α4β2尼古丁乙酰胆碱受占有率研究显示,低剂量(0.5 mg)伐尼克兰即可使丘脑和脑干中α4β2 受体饱和。且在Koegelenberg等[12]对该联合疗法的临床试验中,伐尼克兰联合尼古丁贴剂组与伐尼克兰联合安慰剂组在对吸烟的渴求上无明显差异。二者的研究结果并不支持该假设;(2)尼古丁贴剂可能作用于其他未知受体而在戒烟治疗中发挥作用,α4β2受体的调节可能不是释放多巴胺的唯一途径;或中脑边缘多巴胺回路之外另有尼古丁依赖通路,多巴胺可能不是参与吸烟行为的唯一神经递质;(3)伐尼克兰与尼古丁贴剂药物代谢动力学的差异致更高效受体激动。但在Baker等[14]的临床研究显示,尼古丁贴剂的预处理或延长疗程,并未显著提高戒断率。烟草依赖的形成除前述机制还涉及心理、遗传等众多因素参与[16],伐尼克兰联合尼古丁贴剂的作用机制仍有待进一步探索。
3. 伐尼克兰联合尼古丁贴剂疗效研究
回顾伐尼克兰联合尼古丁贴剂近15年内的临床试验,见表1最早的是Ebbert等[10]的一项回顾性队列研究,虽然其试验结果提示联合疗法无明显获益,但联合用药方案患者可耐受且安全性良好。由于样本量较小,且实验结局指标受主观影响较大,提出联合治疗方案提高疗效的可能。在随后对联合疗法的研究中,结果不一:有两项随机临床对照试验结果提示联合疗法比单药治疗更有效[12,17],三项随机临床对照试验结果提示该联合疗法并无显著获益[11,13−14]。
表 1 伐尼克兰联合尼古丁贴剂临床试验汇总Table 1. Varenicline combined with nicotine patch clinical trial summary临床试验/研究
信息样本量 FTND
评分a纳入人群 用药方案 主要结局指标 不良反应 Koegelenberg[12] 435 4.5 日吸烟量≥10支/日的成人 在TQDb前2周开始尼古丁贴片(15 mg/16 h),并持续12周(NRT总疗程14周)。伐尼克兰在TQD前1周开始使用:第1~3日0.5 mg qd,第4~7日0.5 mg bid,第8日起1 mg bid持续使用12周,并在第13周时减停(伐尼克兰总疗程14周)。 治疗第9~12周经呼出一氧化碳确认(≤10 ppm)的4周持续戒断率 在联合治疗组中,恶心、睡眠障碍、皮肤反应、便秘和抑郁的发生率更高,但只有皮肤反应达到统计学意义(14.4% vs 7.8%, P = 0.03) King[17] 122 3.9 日吸烟量5至30支且酗酒(男性每周饮酒>14杯,女性每周饮酒>7杯,过去一年每月≥1个酗酒日 尼古丁贴片在TQD早晨开始使用,并按照生产商推荐的剂量持续使用 10 周。轻度吸烟者(每日吸烟量<10支):前6 周每天使用 14 mg的贴片,之后 4 周每天使用 7 mg的贴片。重度吸烟者(每日吸烟量≥10 支):前6 周每天使用 21 mg的贴片,之后 2 周每天使用14 mg的贴片,再之后 2 周每天使用7 mg的贴片。伐尼克兰在TQD开始使用:第1~3日0.5 mg qd,第4~7日0.5 mg bid,第8日起1 mg bid持续使用12周,在第13周时减停。 自我报告的治疗第9~12 周持续戒断率(定义为:完全不吸一口烟);第12周时经呼出一氧化碳确认(≤10 ppm戒烟 与单药治疗相比,联合治疗组的参与者出现恶、胀气、梦境异、睡眠问题和头痛的发生率较高 Hajek[11] 117 4.9 成人吸烟者 在TQD当日开始尼古丁贴片(15 mg/16 h),并持续4周。伐尼克兰在TQD前1周开始使用:第1~3日0.5 mg qd,第4~7日0.5 mg bid,第8日起1 mg bid持续使用至第12周(伐尼克兰总疗程13周) 治疗第1~4周经呼出一氧化碳确认(<9 ppm)的4周持续戒断率 在联合治疗组中,梦境异常的发生率相对较高,但未达统计学意义 Ramon[13] 341 6.5 成人且近6个月内日吸烟量≥20支 在TQD当日开始尼古丁贴片(21 mg/24h),并持续12周。伐尼克兰在TQD前1周开始使用:第1~3日0.5 mg qd,第4~7日0.5 mg bid,第8日起1 mg bid持续使用至第12周 治疗第2~12周经呼出一氧化碳确认(<10 ppm)持续戒断率 两组失眠、梦境异常、恶心为最常见不良反应,发生率差异无统计学意义。联合治疗组头痛发生率相对较高,但两组差异无统计学意义 Baker[14] 1251 5.0 成人且过去6个月内日吸烟量≥5支;呼出一氧化碳≥5 ppm 在TQD前2周开始尼古丁贴片(14 mg/24 h),并持续12或24周(NRT总疗程14或26周)。伐尼克兰在TQD前1周开始使用:第1~3日0.5 mg qd,第4~7日0.5 mg bid,第8日起1 mg bid持续使用11或23周(伐尼克兰总疗程12或24周)。 TQD后第52周经呼气一氧化碳验证≤5 ppm的7 天时点戒烟率 在4个治疗组中,最常见的不良事件是恶心、失眠和情绪变化 注:aFTND评分:Fagerstrom尼古丁依赖评估量表评分,0-3分轻度,4-6分中度,7-10分重度;bTQD: Target Quit Day,目标戒烟日 在样本量最大的两项随机临床试验中,标准疗程下联合疗法均采用尼古丁贴剂预处理,但二者所得结论不同,可能原因包括:Koegelenber等[12]的试验中,为规避戒烟药物潜在不良反应,排除了包括慢性阻塞性肺疾病、糖尿病、癌症等在内的多种慢性疾病患者,纳入受试者为相对健康的吸烟者;Koegelenber等[12]的研究中,仅62.3%的受试者完成试验,高失访率可能对结果分析造成影响;Baker等[14]所进行的试验中,伐尼克兰单药治疗12周并无既往临床试验中的“优异表现”,可能未发挥药物的最大效应。
另外三项样本量相对较小且未行尼古丁贴片预处理的临床试验中,King等[17]研究了联合疗法对吸烟同时酗酒人群对的疗效,结果提示联合疗法较单药治疗戒烟率更高,且耐受性良好,为戒烟药物在这一人群的使用提供依据。Hajek等[11]对117名吸烟者进行了随机试验,结果表明联合疗法耐受性良好,但在戒烟方面并不比单独使用伐尼克兰更有效。该试验仅进行短期随访,无法据此得出联合疗法远期无获益结论。Ramon等[13]的研究纳入的受试者吸烟≥20支/d,平均法氏烟草依赖评估量表得分>6分,较其他临床随机对照试验的受试者依赖水平更高。然而在该项研究中联合疗法的戒断率与Hajek等的试验所得相近,组间戒烟率对比同样没有差异。虽然在亚组分析中发现联合疗法在重度吸烟者(≥29支/d)中戒烟率更高,但仍需进一步的研究来证实这一发现。
关于联合疗法的系统回顾及Meta分析显示,与伐尼克兰单药治疗相比,联合疗法可提高疗效,尽管在去除尼古丁贴剂预处理的影响后,两者的差异很小[18]。但该研究仅包含三项随机临床对照试验,样本量最大的试验纳入受试者女性比例更高,用药方案较其余两项不同,结论不可推广。基于可获得的临床证据,美国胸科协会建议对于开始接受治疗的烟草依赖成人患者,推荐使用伐尼克兰而不是尼古丁贴片(强烈推荐,疗效:中等确定)。有条件推荐使用伐尼克兰联合尼古丁贴剂,而不是单独使用伐尼克兰,但该建议估计效果确定性低,并非强力建议[19];美国国家综合癌症网络戒烟指南指出联合疗法可作为被视为一种药物治疗选择,最佳推荐疗法仍为药物治疗(NRT组合:长效贴剂加短效制剂;伐尼克兰单药治疗)联合行为疗法[20],关于伐尼克兰联合尼古丁贴剂的疗效需进一步临床试验才能得出更可靠结论。
伐尼克兰和尼古丁贴剂作为一线戒烟治疗用药,单药治疗疗效已被循证医学证实。而对二者联合治疗的临床试验来看,有提高重度烟草依赖者戒烟成功率的潜力,但在不同研究中表现出的效果不一致,且联合使用可能会增加某些不良反应的发生风险,患者使用药物的依从性下降,经济负担增加,目前指南常规推荐二者分别使用。初步的临床试验提示该联合治疗方案延长疗程下亦无显著疗效。进一步的研究可选择其他类型的尼古丁替代药物联合伐尼克兰,或其他类型的戒烟药物联合伐尼克兰,如安非他酮等,探究不同组合疗法的优势,确定是否可以联合使用这些药物以及最佳的联合治疗方案。如Gregory R Weeks等[21]选择了伐尼克兰联合尼古丁含片(相对尼古丁贴剂速效)对比伐尼克兰单药的疗效进行初步研究,虽然经呼出气一氧化碳验证(FeCO ≤ 6 ppm)的戒烟率并无提高,但其安全性良好。现有研究设计存在差异、随访时间较短,导致研究结果存在一定的异质性。且目前国内关于伐尼克兰联合尼古丁贴剂的研究相对较少,缺乏国人的临床研究数据,未来,需要开展更多大样本、多中心、随机对照的临床试验,进一步验证伐尼克兰联合尼古丁贴剂的疗效和安全性。同时,应深入研究联合治疗的最佳方案,包括药物剂量、治疗疗程、适用人群等,以提高治疗效果和患者的依从性。此外,结合行为干预、心理辅导等综合戒烟措施,可能会进一步提高戒烟成功率,为吸烟者提供更有效的戒烟治疗策略。
4. 伐尼克兰联合尼古丁贴剂安全性
在上述采用联合疗法的临床试验中,联合疗法组发生胃肠道反应、梦境异常的风险较单药治疗略高,但在受试者可耐受范围内,很少导致戒烟治疗的终止。仅在一项研究中发现联合疗法可能增加皮肤不良反应发生风险,皮肤反应在联合治疗后增加(14.4% vs 7.8%; P = 0.03) [12],这可能是由于两种药物的叠加作用导致的。虽然现有研究证据未提示联合疗法显著增加不良反应发生风险,大多数不良反应较轻且可耐受,但长期安全性研究仍待探索。且目前对该疗法的研究集中于相对健康的吸烟者,对部分特殊人群,如精神障碍患者、心血管疾病患者、呼吸道疾病患者的安全性仍缺乏研究,在这部分人群中,戒烟是可获益的干预因素,需随访时间足够长的高质量证据为联合疗法的应用提供依据。由于孕妇的特殊性,目前缺乏关于妊娠人群戒烟药物有效性的明确证据,对现有的临床证据分析,尼古丁替代疗法在对不良妊娠结局的潜在影响不明确[22],对于对尝试戒烟失败的孕妇,在充分告知风险并取得同意后尝试中使用尼古丁替代疗法[23],伐尼克兰和安非他酮在孕妇中的使用证据非常少,不推荐在妊娠人群中使用。
5. 小结
烟草成瘾者对尼古丁依赖程度不同,个体差异较大,针对吸烟者的需求和特点,对于重度依赖者往往采取包括心理、行为干预、戒烟药物联合等在内的戒烟联合干预手段,目前尚无有力临床证据表明该联合疗法有显著获益,未来还需收集更多临床应用数据,为该疗法合理、有效治疗烟草依赖提供依据。
-
表 1 RT-qPCR扩增引物序列表
Table 1. Nucleotide sequences of the primers used for real-time quantitative PCR
引物名称 引物序列 miR-490-3p forward 5′-CGTGGATCCTTCTTCAACCAACGGTGGTG-3′ miR-490-3p reverse 5′-CCAGAATTCAAAGCAGGAAGAGTAAGACTTCC-3′ U6 forward 5′-GCTTCGGCAGCACATATACTAA-3′ U6 reverse 5′-CGAATTTGCGTGTCATCCTT-3′ -
[1] Torre L A,Bray F,Siegel R L,et al. Global cancer statistics,2012[J]. CA:a Cancer Journal for Clinicians,2015,65(2):87-108. doi: 10.3322/caac.21262 [2] Ansari D,Tingstedt B,Andersson B,et al. Pancreatic cancer:Yesterday,today and tomorrow[J]. Future Oncology (London,England),2016,12(16):1929-1946. doi: 10.2217/fon-2016-0010 [3] 许伟先,何志伟,喻超,等. microRNA-125b对胰腺癌侵袭转移的影响及其机制[J]. 贵阳医学院学报,2019,44(8):881-885. [4] Khan S,Anasrullah,Kumar D,et al. Targeting microRNAs in pancreatic cancer:microplayers in the big game[J]. Cancer Research,2013,73(22):6541-6547. doi: 10.1158/0008-5472.CAN-13-1288 [5] Aiello N M,Brabletz T,Kang Y,et al. Upholding a role for EMT in pancreatic cancer metastasis[J]. Nature,2017,547(7661):E7-E8. doi: 10.1038/nature22963 [6] Beuran M,Negoi I,Paun S,et al. The epithelial to mesenchymal transition in pancreatic cancer:A systematic review[J]. Pancreatology:Official Journal of the International Association of Pancreatology (IAP),2015,15(3):217-225. doi: 10.1016/j.pan.2015.02.011 [7] Gaianigo N,Melisi D,Carbone C. EMT and treatment resistance in pancreatic cancer[J]. Cancers,2017,9(9):122-138. [8] Zhou P,Li B,Liu F,et al. The epithelial to mesenchymal transition (EMT) and cancer stem cells:implication for treatment resistance in pancreatic cancer[J]. Molecular Cancer,2017,16(1):52-63. doi: 10.1186/s12943-017-0624-9 [9] 王甫珏,李君君,谢海涛,等. 地西他滨对 K562 细胞增殖和 TFPI-2 基因表达的影响[J]. 中华血液学杂志,2017,38(4):340-343. doi: 10.3760/cma.j.issn.0253-2727.2017.04.016 [10] Moore A,Donahue T. Pancreatic cancer[J]. Jama,2019,322(14):1426-1426. doi: 10.1001/jama.2019.14699 [11] Beger H G, Birk D. Pancreatic cancer staging systems and their clinical impact[M]. Hoboken, New Jersey, USA: John Wiley & Sons, Ltd, 2009: 25-158. [12] Xu B,Liu J,Xiang X,et al. Expression of miRNA-143 in pancreatic cancer and its clinical significance[J]. Cancer Biotherapy & Radiopharmaceuticals,2018,33(9):373-379. [13] Shang S,Wang J,Chen S,et al. Exosomal miRNA-1231 derived from bone marrow mesenchymal stem cells inhibits the activity of pancreatic cancer[J]. Cancer Medicine,2019,8(18):7728-7740. doi: 10.1002/cam4.2633 [14] 邓思远,贺德,马广念,等. 微小RNA-490-3p对人胰腺癌的增殖,迁移,侵袭,凋亡的影响[J]. 中华实验外科杂志,2020,37(4):673-675. doi: 10.3760/cma.j.cn421213-20190715-01002 [15] Ou Y,He J,Liu Y. MiR-490-3p inhibits autophagy via targeting ATG7 in hepatocellular carcinoma[J]. IUBMB Life,2018,70(6):468-478. doi: 10.1002/iub.1715 [16] Fan H,Zhang Y S. miR-490-3p modulates the progression of prostate cancer through regulating histone deacetylase 2[J]. European Review for Medical and Pharmacological Sciences,2019,23(2):539-546. [17] Liu X,He B,Xu T,et al. MiR-490-3p functions as a tumor suppressor by inhibiting oncogene VDAC1 expression in colorectal cancer[J]. Journal of Cancer,2018,9(7):1218-1230. doi: 10.7150/jca.23662 [18] Zhang F,Wu A,Wang Y,et al. miR-490-3p functions as a tumor suppressor in glioma by inhibiting high-mobility group AT-hook 2 expression[J]. Experimental and Therapeutic Medicine,2019,18(1):664-670. [19] De Craene B,Berx G. Regulatory networks defining EMT during cancer initiation and progression[J]. Nature Reviews Cancer,2013,13(2):97-110. doi: 10.1038/nrc3447 [20] 邹晨. Prrx1基因在胰腺癌EMT中的作用及机制研究[D], 南京: 南京医科大学博士学位论文, 2016. [21] 朱亮. RGC-32在胰腺癌EMT中的作用及机制研究[D]; 武汉: 华中科技大学博士学位论文, 2012. [22] 邵珊,秦涛,钱伟琨,等. Hedgehog通路通过非配体依赖途径调控缺氧诱导的胰腺癌EMT及侵袭过程[J]. 西安交通大学学报(医学版),2020,41(3):347-355. [23] Krebs A M,Mitschke J,Lasierra Losada M,et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer[J]. Nature Cell Biology,2017,19(5):518-529. doi: 10.1038/ncb3513 [24] Fusco A,Fedele M. Roles of HMGA proteins in cancer[J]. Nature Reviews Cancer,2007,7(12):899-910. doi: 10.1038/nrc2271 [25] Watanabe S,Ueda Y,Akaboshi S,et al. HMGA2 maintains oncogenic RAS-induced epithelial-mesenchymal transition in human pancreatic cancer cells[J]. The American Journal of Pathology,2009,174(3):854-868. doi: 10.2353/ajpath.2009.080523 [26] Zhang S,Mo Q,Wang X. Oncological role of HMGA2 (Review)[J]. International Journal of Oncology,2019,55(4):775-788. [27] Hawsawi O,Henderson V,Burton L J,et al. High mobility group A2 (HMGA2) promotes EMT via MAPK pathway in prostate cancer[J]. Biochemical and Biophysical Research Communications,2018,504(1):196-202. doi: 10.1016/j.bbrc.2018.08.155 [28] Dong J,Wang R,Ren G,et al. HMGA2-FOXL2 axis regulates metastases and epithelial-to-mesenchymal transition of chemoresistant gastric cancer[J]. Clinical Cancer Research:an Official Journal of the American Association for Cancer Research,2017,23(13):3461-3473. doi: 10.1158/1078-0432.CCR-16-2180 期刊类型引用(2)
1. 周炜,耿娜薇,王颖,臧伟,刘丙木,曹莉明. CT引导肝癌微波消融术后CT灌注参数与近期疗效及预后的关系研究. 中华全科医学. 2024(10): 1742-1745+1786 .  百度学术
百度学术2. 周永祥,赵振国,张景俊,王苑植,郭于风,首峰. 基于CT和磁共振成像人工智能图像融合技术的适形消融用于肝癌TACE术后残留的疗效分析. 临床误诊误治. 2024(21): 67-72+78 .  百度学术
百度学术其他类型引用(0)
-






 下载:
下载:
 下载:
下载: