Effect of Ginsenoside Rg1 on Renal Protection in Type 2 Diabetic Rats
-
摘要:
目的 探讨人参皂苷Rg1对2型糖尿病大鼠肾脏的保护作用及机制。 方法 采用高糖高脂饮食 + 小剂量链脲佐菌素(STZ)建立2型糖尿病大鼠模型,给予人参皂苷Rg1灌胃4周治疗后,测定血肌酐(Scr)、尿素氮(BUN)、尿酸(UA)。取肾脏组织切片进行HE染色,观察肾脏组织的病理改变;用Westernblot检测大鼠肾脏组织内血管紧张素Ⅱ1型受体(AT1)、转化生长因子-β1(TGF-β1)、金属蛋白酶-9(MMP-9)的表达水平。 结果 2型糖尿病大鼠BUN明显高于正常组,但是Scr、UA与正常组比无统计学意义(P > 0.05)。人参皂苷Rg1不同剂量治疗后BUN值下降,二甲双胍组BUN也下降,而Scr、UA变化差异无统计学意义( P > 0.05)。糖尿病大鼠肾系数高于正常组( P < 0.05),给与人参皂苷Rg1以及二甲双胍治疗后肾体比下降( P < 0.05)。糖尿病大鼠肾脏AT1及TGF-β 1表达高于正常组(P < 0.05),给与人参皂苷Rg1不同剂量以及二甲双胍治疗后表达降低( P < 0.05)。MMP9在糖尿病大鼠肾脏中表达下降,而在人参皂苷Rg1以及二甲双胍治疗后表达增加。 结论 人参皂苷Rg1能够保护大鼠肾脏受损,其分子机制可能是通过抑制RAS系统激活,抑制TGF-β1的表达,促进细胞外基质的水解,减轻肾脏受损程度。 -
关键词:
- 人参皂苷Rg1 /
- 2型糖尿病 /
- 肾脏RAS系统 /
- 对肾的保护作用及机制
Abstract:Objective To investigate the protective effect and mechanism of ginsenoside Rg1 on the kidneys of type 2 diabetic rats. Methods A high-sugar and high-fat diet + low-dose streptozotocin (STZ) was used to establish a type 2 diabetic rat model. Ginsenoside Rg1 was administered to the stomach for 4 weeks, and blood creatinine (Scr)、uric acid (UA) and urea nitrogen (BUN) were measured. Take kidney tissue sections for HE staining to observe the pathological changes of kidney tissue; Western blot was used to detect angiotensin Ⅱ type 1 receptor (AT1), transforming growth factor-β1 (TGF-β1) and metalloproteinase-9 in rat kidney tissue expression level. Results BUN of type 2 diabetic rats was significantly higher than that of the normal group, but Scr and UA were not statistically significant compared with the normal group. After different doses of ginsenoside Rg1, the BUN value decreased, and the BUN of the metformin group also decreased, but there was no statistical difference in the changes of Scr and UA (P > 0.05). The renal coefficient of diabetic rats was higher than that of the normal group, and the renal body ratio decreased after treatment with ginsenoside Rg1 and metformin ( P < 0.05). The expressions of AT1 and TGF-β1 in the kidneys of diabetic rats were higher than those in the normal group, and the expressions decreased after being given different doses of ginsenoside Rg1 and metformin treatment ( P < 0.05). The expression of MMP9 decreased in the kidneys of diabetic rats, but increased after treatment with ginsenoside Rg1 and metformin ( P < 0.05). Conclusions Ginsenoside Rg1 can protect the kidney from damage in rats. Its molecular mechanism may be through inhibiting the activation of the RAS system, inhibiting the expression of TGF-β1, promoting the hydrolysis of extracellular matrix, and reducing the degree of kidney damage. -
2型糖尿病(Type 2 diabetes mellitus,T2DM)是由多种因素导致血糖升高的代谢紊乱性疾病,胰岛素抵抗是其主要诱因[1],肾脏损害是糖尿病严重的并发症,也是临床上终末期肾病的首位病因[2]。因此,阐明T2DM合并肾脏损伤的机制,寻找有效的药物治疗成为当前的重要问题。
肾素血管紧张素系统(renin angiotensin s-ystem,RAS)在T2DM及其并发症的发生和发展中具有重要的地位,RAS的激活可增加胰岛素抵抗,促进炎性反应,引起肾炎[3]。血管紧张素Ⅱ 1型受体(angiotensin Ⅱ receptor 1 type, AT1)的表达增加可引起TGF-β1的生成增多,激活RAS导致糖尿病肾病的发生发展[4]。另外,糖尿病患者肾脏中肾小球硬化导致肾功能减退,基质金属蛋白酶9(matrix metalloproteinase, MMP9)通过水解细胞外基质,减轻糖尿病肾脏的恶化[5-7]。所以,抑制RAS的激活和增加细胞外基质的水解将是治疗的关键。
人参皂苷Rg1是我国传统名贵药材人参和三七等主要活性物质[8]。具有抗衰老[9]、抗心肌损伤[10-12]、减缓认知功能障碍[13]、减轻糖尿病并发症如肝损伤[14]、勃起功能障碍[15]的功能。但在T2DM合并肾脏损伤中,人参皂苷Rg1是否抑制RAS的激活和促进细胞外基质的降解,从而保护肾脏的功能未见报道,因此,本研究拟通过建立T2DM的动物模型,探讨人参皂苷Rg1对肾脏组织RAS的激活和促进细胞外基质的降解影响,为人参皂苷Rg1治疗T2DM合并肾脏损伤提供理论依据。
1. 材料与方法
1.1 动物模型及分组
SPF级雄性 SD 大鼠60只(实验所涉及到的实验动物程序均经昆明医科大学实验动物伦理委员会批准,动物合格证号:53004100000079),体重 180~200 g,适应性喂养2周后,分为正常对照组10只,高糖高脂组50只。高糖高脂组喂养高糖高脂饲料8周后,35 mg/kg腹腔注射链脲佐菌素(streptozocin,STZ)1次,1周后测空腹血糖,血糖 ≥ 11.1 mmol/L为造模成功;选择造模成功大鼠分为模型对照组、Rg1低剂量治疗组(25 mg/kg·d)、Rg1中剂量治疗组(50 mg/kg·d)、Rg1高剂量治疗组(100 mg/kg·d)和二甲双胍对治疗组(100 mg/kg·d),每组10只,灌胃给药4周。
1.2 取材
灌胃结束后,以10%水合氯醛麻醉大鼠(6.25 mL/kg),取血离心获得血清后于-80 ℃保存用于检测生化指标;取部分左侧肾脏组织4%甲醛固定后,石蜡包埋用于HE染色,剩余肾脏组织于-80 ℃冰箱保存,用于Western blot检测蛋白质表达。
1.3 血生化指标检测
全自动生化分析仪测定Scr、BUN和UA含量。
1.4 肾体比检测
各组大鼠麻醉前,称取其体重。麻醉处死后取左侧肾脏称取体重,计算肾重量/体重的比值。
1.5 HE染色检测肾脏病理改变
取石蜡包埋后的肾脏组织,通过切片机将组织切为5 µm薄片后经过烤片,二甲苯脱蜡30 min,95%酒精-50%酒精各浸泡2 min,苏木精染色5 min,流水冲洗30 min放入氨水中4 s。1%伊红浸泡3 min,再进行脱水后用中性树脂封片,显微镜下一个样本随机选取6个视野采集图片。
1.6 Western blot检测
从-80 ℃取出肾脏组织称取100 mg,在EP管中加入组织裂解液1 mL,提取总蛋白,采用BCA蛋白定量法定量。取40 µg的蛋白质经SDS-PAGE电泳后采用湿转法把蛋白转移至PVDF膜上,转膜完成后5%脱脂牛奶封闭2 h,加入一抗AT1(Wanleibio)、TGF-β1(Abcam)、MMP9(Wanleibio)和β-actin(Abcam)(1∶1000),孵育过夜后,PBS洗3次,二抗孵育2 h后洗涤,加入ECL发光液,置于凝胶成像系统中进行曝光,保存图片。采用 ImagePro-plus6.0软件进行灰度分析,以β-actin作为内参,分析结果。
1.7 统计学处理
通过SPSS25.0进行统计学处理,计量资料服从正态分布的以均数±标准差( $\bar x\pm s $)表示,组间比较采用单因素方差分析法,P < 0.05为差异有统计学意义,应用GraphPad Prism6.0版进行图片制作。
2. 结果
2.1 生化指标检测
与正常组比较,模型组血BUN增加(P < 0.001),与模型组比较,低、中剂量Rg1组大鼠BUN降低( P < 0.01)。与正常组比较,模型组Scr改变无统计学意义,不同剂量Rg1治疗组与模型组相比无统计学差异。UA值在正常组、模型组大鼠以及Rg1不同治疗组中的改变均无统计学意义,见 表1。
表 1 血生化指标( $\bar x \pm s $)Table 1. Blood biochemical index ( $\bar x \pm s $)检测指标 正常组(n = 6) 模型组(n = 6) 低剂量Rg1(n = 6) 中剂量Rg1(n = 6) 高剂量Rg1(n = 6) 二甲双胍(n = 6) 尿素氮 4.93 ± 0.562 10.00 ± 0.628# 6.41 ± 0.509* 7.74 ± 0.296* 7.18 ± 0.614* 12.97 ± 0.848* 肌酐 41.67 ± 3.667 43.22 ± 2.253 44.86 ± 2.865 42.22 ± 1.913 48.22 ± 3.643 50.33 ± 2.744 尿酸 5.50 ± 0.269 5.00 ± 0.289 5.13 ± 0.350 5.89 ± 0.824 6.00 ± 0.373* 5.75 ± 0.366 与正常组相比较具有统计学意义,#P < 0.05;与模型组比较有统计学意义, *P < 0.05。 2.2 左肾重、肾体积以及肾体比
与正常组比较,模型组左侧肾脏的重量增加(P < 0.05),与模型组比较,各剂量Rg1组和二甲双胍组治疗后左肾重减轻( P < 0.05)。与正常组比较,模型组大鼠体重降低( P < 0.05),低、中、高3个剂量Rg1和二甲双胍治疗后体重与模型组相比无统计学意义。与正常组大鼠相比,模型组大鼠肾脏系数增大,提示可能有肾脏充血、水肿或是肥大增生。给与Rg1以及二甲双胍治疗后肾脏系数降低( P < 0.05),见 表2。
表 2 肾脏指标( $\bar x \pm s $)Table 2. Kidney indicators ( $\bar x \pm s $)检测指标 正常组(n = 6) 模型组(n = 6) 低剂量Rg1(n = 6) 中剂量Rg1(n = 6) 高剂量Rg1(n = 6) 二甲双胍(n = 5) 左肾重 1.84 ± 0.131 2.22 ± 0.082# 1.90 ± 0.052* 1.43 ± 0.114* 1.58 ± 0.091* 1.90 ± 0.110* 体重 570.50 ± 17.480 376.80 ± 7.016# 395.30 ± 11.070 366.50 ± 4.403 367.00 ± 8.450 398.20 ± 9.932 肾重/体重 0.32 ± 0.022 0.59 ± 0.024# 0.48 ± 0.008* 0.39 ± 0.030* 0.43 ± 0.028* 0.48 ± 0.021* 与正常组比较具有统计学意义,#P < 0.05;与模型组比较有统计学意义, *P < 0.05。 2.3 肾脏HE染色结果
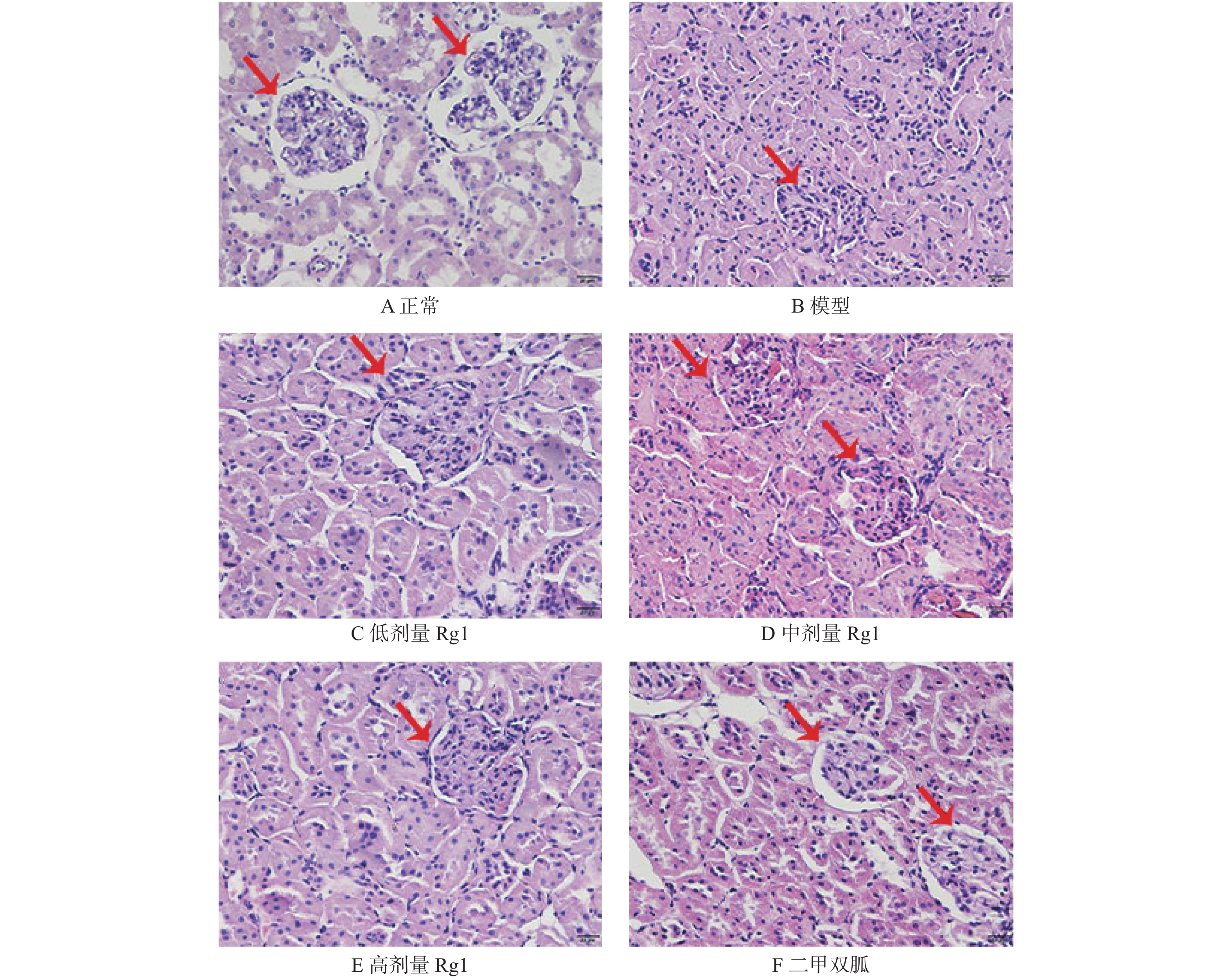
与正常组相比,模型组大鼠的肾脏肾小球体积增大、细胞数目增多。系膜基质增多,系膜区增宽。炎性细胞浸润,肾小囊消失肾小管上皮细胞出现水肿以及坏死脱落。给与各剂量Rg1和二甲双胍治疗后,肾小球体积减小,炎性细胞浸润程度下降,肾小囊部分可见,见图1。
2.4 肾脏Western blot检测结果
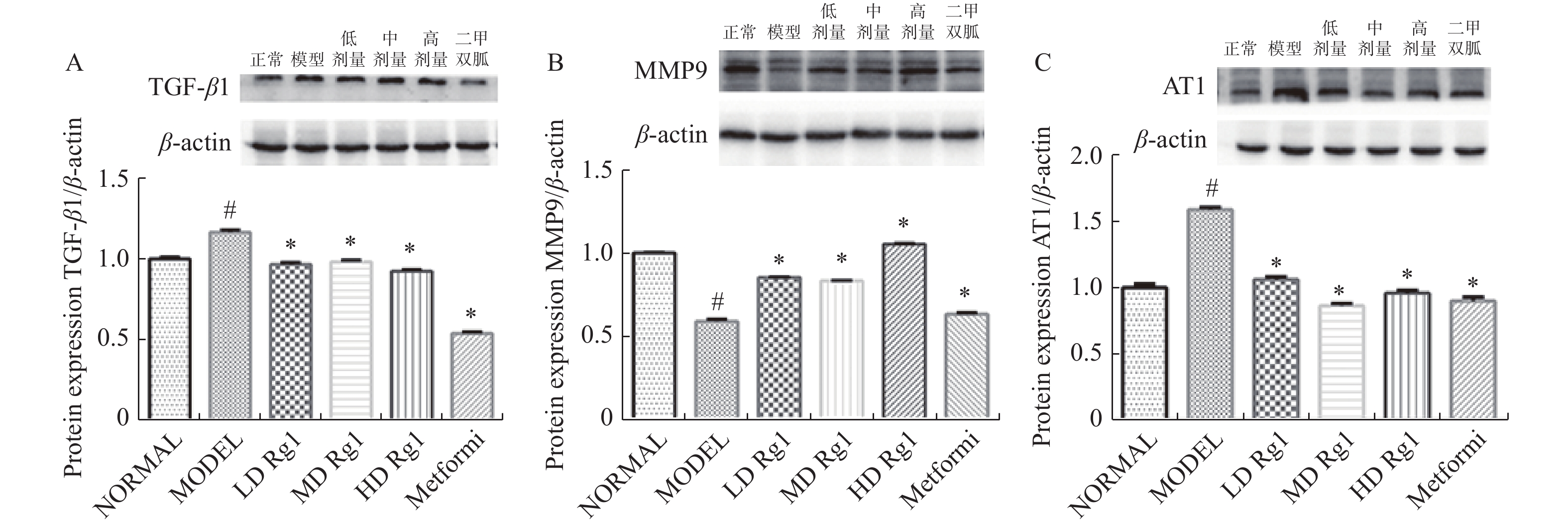
与正常组相比,模型组肾脏组织TGF-β1分泌增多,模型组与正常组相比有统计学意义P < 0.05,给与Rg1不同剂量和二甲双胍治疗后TGF-β1分泌减少,剂量组和模型组相比有统计学意义 P < 0.05。MMP9在糖尿病模型组大鼠肾脏中的表达低于正常组,模型组与正常组相比有统计学意义 P < 0.05。给与Rg1不同剂量和二甲双胍治疗后,MMP9的表达较模型组相比升高,有统计学意义 P < 0.05。AT1在糖尿病大鼠模型组肾脏中的表达高于模型组,模型组与正常组相比有统计学意义 P < 0.05。给与Rg1不同剂量以及二甲双胍治疗后AT1表达下降明显,接近正常组,Rg1不同剂量治疗组以及二甲双胍组与模型组相比具有统计学意义 P < 0.05,见 图2。
3. 讨论
杨敬等[16]研究发现人参皂苷Rg1可显著改善肾损伤大鼠血清氧化应激指标,下调血清炎性因子水平以及肾组织TGF-β1、MCP-1 mRNA表达。沈晓燕等研究发现Rg1可通过抑制NOX4介导的ROS氧化应激和NLRP3炎症小体激活来延缓肾脏衰老,减轻与衰老相关的肾损伤和纤维化[17]。在本研究中从血生化数据中可看出采用高糖高脂饮食 + 小剂量链脲佐菌素(STZ)建立的2型糖尿病大鼠BUN值显著升高,而尿酸值以及肾脏系数指标下降,本研究从大体角度对人参皂苷Rg1治疗肾脏损伤的表型进行了描述,说明人参皂苷Rg1可以保护大鼠肾脏受损。这与其他研究结论一致[18-20]。
陈少霞等研究发现局部肾素血管紧张素系统的过度激活引起肾脏足细胞、内皮细胞、系膜细胞、肾小管上皮细胞的损害,导致肾脏病理改变,引起一系列临床表现。其他研究也证明了肾素血管紧张素系统的过度激活可能会引起肾脏的损伤[21-22],肾素血管紧张素系统的激活表现为血管紧张素受体AT1表达增加[23],使用AT1抑制剂可以降低糖尿病大鼠肾脏受损程度[24],本研究免疫印迹结果显示糖尿病大鼠肾脏中AT1表达增加,说明RAS系统的激活参与了糖尿病大鼠肾脏受损的分子调节。
糖尿病早期肾损害表现为系膜细胞增殖、大量细胞外基质生成并异常聚集,TGF-β1与系膜细胞增殖有关,早期2型糖尿病TGF-β1分泌升高[25-26]。本研究免疫印迹结果显示糖尿病大鼠肾脏中TGF-β1表达增高,这可能是由于RAS系统激活所致。MMP9在糖尿病大鼠肾脏表达减少,糖尿病大鼠肾脏中细胞外基质ECM不能被正常代谢从而促进肾脏受损。人参皂苷Rg1保护糖尿病大鼠肾脏受损的分子机制可能是Rg1抑制RAS系统激活,抑制TGF-β1的表达,从而促进ECM的水解,减缓肾脏受损程度。
根据本实验研究结果分析发现,人参皂苷Rg1可有效缓解2型糖尿病大鼠的症状,减少糖尿病大鼠的肾脏损伤,其作用机制是Rg1对RAS系统的作用,调控了炎性因子的表达,从而实现对肾脏功能的良性调节。人参皂苷Rg1可以作为2型糖尿病引起肾脏损伤的预防药物,但其作用机制有待更深入研究。
-
表 1 血生化指标( $\bar x \pm s $)
Table 1. Blood biochemical index ( $\bar x \pm s $)
检测指标 正常组(n = 6) 模型组(n = 6) 低剂量Rg1(n = 6) 中剂量Rg1(n = 6) 高剂量Rg1(n = 6) 二甲双胍(n = 6) 尿素氮 4.93 ± 0.562 10.00 ± 0.628# 6.41 ± 0.509* 7.74 ± 0.296* 7.18 ± 0.614* 12.97 ± 0.848* 肌酐 41.67 ± 3.667 43.22 ± 2.253 44.86 ± 2.865 42.22 ± 1.913 48.22 ± 3.643 50.33 ± 2.744 尿酸 5.50 ± 0.269 5.00 ± 0.289 5.13 ± 0.350 5.89 ± 0.824 6.00 ± 0.373* 5.75 ± 0.366 与正常组相比较具有统计学意义,#P < 0.05;与模型组比较有统计学意义, *P < 0.05。 表 2 肾脏指标( $\bar x \pm s $)
Table 2. Kidney indicators ( $\bar x \pm s $)
检测指标 正常组(n = 6) 模型组(n = 6) 低剂量Rg1(n = 6) 中剂量Rg1(n = 6) 高剂量Rg1(n = 6) 二甲双胍(n = 5) 左肾重 1.84 ± 0.131 2.22 ± 0.082# 1.90 ± 0.052* 1.43 ± 0.114* 1.58 ± 0.091* 1.90 ± 0.110* 体重 570.50 ± 17.480 376.80 ± 7.016# 395.30 ± 11.070 366.50 ± 4.403 367.00 ± 8.450 398.20 ± 9.932 肾重/体重 0.32 ± 0.022 0.59 ± 0.024# 0.48 ± 0.008* 0.39 ± 0.030* 0.43 ± 0.028* 0.48 ± 0.021* 与正常组比较具有统计学意义,#P < 0.05;与模型组比较有统计学意义, *P < 0.05。 -
[1] Khan M A B. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends[J]. Journal of epidemiology and global health,2020,10(1):107-111. [2] 吴雪怡. 2型糖尿病肾损害的病理分型及其临床意义[D]. 北京: 北京协和医学院博士论文, 2012. [3] 赵莹莹. 肾素-血管紧张素系统与糖尿病[J]. 中国组织工程研究,2014,018(B12):92-92. [4] Nakamura T,Obata J,Kimura H,et al. Blocking angiotensin Ⅱ ameliorates proteinuria and glomerular lesions in progressive mesangioproliferative glomerulonephritis[J]. Kidney international,1999,55(3):877-889. doi: 10.1046/j.1523-1755.1999.055003877.x [5] 董骏武,刘晓城,刘慎微,等. 羟苯磺酸钙对糖尿病大鼠肾小球MMP9/TIMP1的影响[J]. 临床内科杂志,2005,22(6):419-421. doi: 10.3969/j.issn.1001-9057.2005.06.022 [6] 马洪波. MMP—9在糖尿病肾病中的研究进展[J]. 国外医学(老年医学分册),2003,(3):114-118. [7] 王永强. 雷帕霉素对STZ致糖尿病幼年大鼠肾脏NF-κB与MMP9表达的影响[M]. 南昌: 南昌大学硕士论文, 2014. [8] 郭从亮,崔秀明,杨晓艳,等. 人参皂苷生物转化研究进展[J]. 中国中药杂志,2014,39(20):3899-3904. [9] 李成鹏,张梦思,刘俊,等. 人参皂苷Rg_1延缓脑衰老机制研究[J]. 中国中药杂志,2014,39(22):4442-4447. [10] 尉海涛. 人参皂苷Rg1对糖尿病大鼠心肌损伤的保护作用及机制研究[M]. 长春: 吉林大学硕士论文, 2016. [11] 詹淑玉,邵青,李正,等. 生脉注射液中人参皂苷Rg_1,Rb_1在心肌缺血大鼠体内的药动学-药效学结合研究[J]. 中国中药杂志,2014,39(7):1300-1305. [12] 张庆勇,陈燕萍,刘芬,等. 人参皂苷Rg1对大鼠急性心肌缺血抗氧化损伤指标及超微结构的影响[J]. 中国循环杂志,2015,30(2):164-167. doi: 10.3969/j.issn.1000-3614.2015.02.017 [13] 张潇丹. 人参皂苷Rg1对阿尔茨海默病大鼠认知功能障碍的作用及机制研究[M]. 济南: 山东大学硕士论文, 2013. [14] 陈羡人,高雅文,邓小梅. 人参皂苷Rg1对糖尿病大鼠肝损伤、氧化应激及肝组织Toll样受体4表达的影响[J]. 中国临床药理学杂志,2020,36(2):146-149. [15] 夏雨果,陈秋,高坪,等. 人参皂苷Rg_1对糖尿病大鼠勃起功能障碍的影响[J]. 中成药,2019,41(6):1258-1263. doi: 10.3969/j.issn.1001-1528.2019.06.012 [16] 杨敬,代俞燕,熊燕影等. 人参皂苷Rg1对糖尿病肾病大鼠血清氧化应激指标、炎性因子及肾组织TGF-β1、MCP-1 mRNA的影响[J]. 现代生物医学进展,2020,20(5):853-856+918. [17] 沈晓燕. NOX4-NLRP3信号通路在小鼠肾脏老化损伤中的作用及人参皂苷Rg1的调节作用[D]. 安徽医科大学硕士论文, 2020. [18] 黄琦, 倪海祥, 苏建明. 人参皂苷对2型糖尿病大鼠肾组织基质金属蛋白酶的影响[C]. 浙江省内分泌学学术会议, 2008, p2, 中国浙江临海. [19] 倪海祥,杨雪辉,朱峰,等. 人参皂苷对糖尿病大鼠肾组织基质金属蛋白酶2表达的影响[J]. 中国中西医结合肾病杂志,2009,10(3):211-213. doi: 10.3969/j.issn.1009-587X.2009.03.008 [20] 苏建明,倪海祥,黄琦,等. 人参皂苷对糖尿病大鼠肾组织基质金属蛋白酶9及Ⅳ型胶原表达的影响[J]. 中国中医急症,2011,20(6):924-925. doi: 10.3969/j.issn.1004-745X.2011.06.037 [21] 陈少霞,龚莉. 肾脏局部肾素血管紧张素系统与糖尿病肾病肾脏细胞的损害[J]. 糖尿病新世界,2016,19(2):115-117. [22] 侯丽娜,毕力夫,苏秀兰. RAS基因多态性与糖尿病合并高血压相关性研究进展[J]. 中国医药导报,2017,14(23):33-35. [23] 马也娉,张铮,卓莉,等. 肾活检组织血管紧张素AT1、AT2和MAS受体表达与糖尿病肾病病理损伤的关系[J]. 国际泌尿系统杂志,2018,38(2):256-261. doi: 10.3760/cma.j.issn.1673-4416.2018.02.023 [24] 张芳. ACEI联合AT1受体阻断剂双重阻断RAS系统治疗老年糖尿病肾病疗效分析[J]. 蚌埠医学院学报,2020,45(1):74-77. [25] 包慧兰,叶赏和,楼时先,等. 早期糖尿病肾病血清HGF,Cys C和TGF-β1水平及平肾汤干预的影响[J]. 中国中药杂志,2014,39(6):1128-1131. [26] 张倩. 糖尿病肾病患者TGF-β1基因表达调控区甲基化水平变化及意义的研究[M]. 重庆: 第三军医大学硕士论文, 2016. -






 下载:
下载:
 下载:
下载:

