Effect of Intestinal Flora Metabolites Deoxycholic Acid on the Proliferation and Cell Cycle of Human Umbilical Cord Mesenchymal Stem Cells
-
摘要:
目的 探讨肠道菌群代谢物次级胆汁酸DCA不同浓度和不同作用时间在体外环境下对人脐带间充质干细胞(human umbilical cord mesenchymal stem cells,hUC-MSCs)增殖和细胞周期的影响。 方法 CCK8及流式细胞仪检测在含不同浓度DCA(0.00 μmol/L、1.56 μmol/L、3.13 μmol/L、6.25 μmol/L、12.50 μmol/L、25.00 μmol/L、50.00 μmol/L、100.00 μmol/L、200.00 μmol/L、400.00 μmol/L、800.00 μmol/L)干细胞培养基中培养hUC-MSCs 24 h、48 h、72 h后,干细胞增殖及细胞周期的变化。 结果 脱氧胆酸对hUC-MSCs增殖的影响体现在DCA剂量和作用时间两方面(两者交互作用,F = 6.622,P < 0.001);当浓度一定时,时间越长,细胞增殖越多;当作用时间一定时,浓度越高,细胞增殖抑制。浓度一定时,随时间的增加,细胞阻滞在G0/G1期的比例增加(P < 0.001);不同作用时间DCA对 hUC-MSCs的细胞周期表现为低浓度组细胞阻滞在G0/G1期和S期,高浓度组细胞阻滞在S期和G2/M期(P < 0.001)。 结论 体外环境下,DCA对hUC-MSCs增殖及细胞周期的影响与浓度和作用时间相关。 Abstract:Objective To investigate the influence of intestinal flora metabolites secondary bile acid DCA at different concentration and reaction time on the proliferation and cell cycle of human umbilical cord mesenchymal stem cells (hUC-MSCs) in vitro. Methods CCK8 and flow cytometry were used to detect the changes in cell proliferation and cell cycle of hUC-MSCs cultured in stem cell medium containing different concentrations of DCA (0.00 μmol/L, 1.56 μmol/L, 3.13 μmol/L, 6.25 μmol/L, 12.50 μmol/L, 25.00 μmol/L, 50.00 μmol/L, 100.00 μmol/L, 200.00 μmol/L, 400.00 μmol/L, 800.00 μmol/L) at 24h, 48h and 72h. Results The effect of deoxycholic acid on the proliferation of hUC-MSCs was both dose- and time-dependent (interaction, F = 6.622, P < 0.001).When the concentration was constant, cell proliferation increases with time; When the treatment time was fixed, the cell proliferation decreased with the increase of concentration. When the concentration was constant, the proportion of cell was arrested in G0/G1 phase increased with time (P < 0.001).The cell cycle of hUC-MSCs treated by DCA was blocked in G0/G1 and S phase in low concentration group, while S and G2/M phase in high concentration group (P < 0.001). Conclusion The effects of DCA on the proliferation and cell cycle of hUC-MSCs were both dose and time in vitro. -
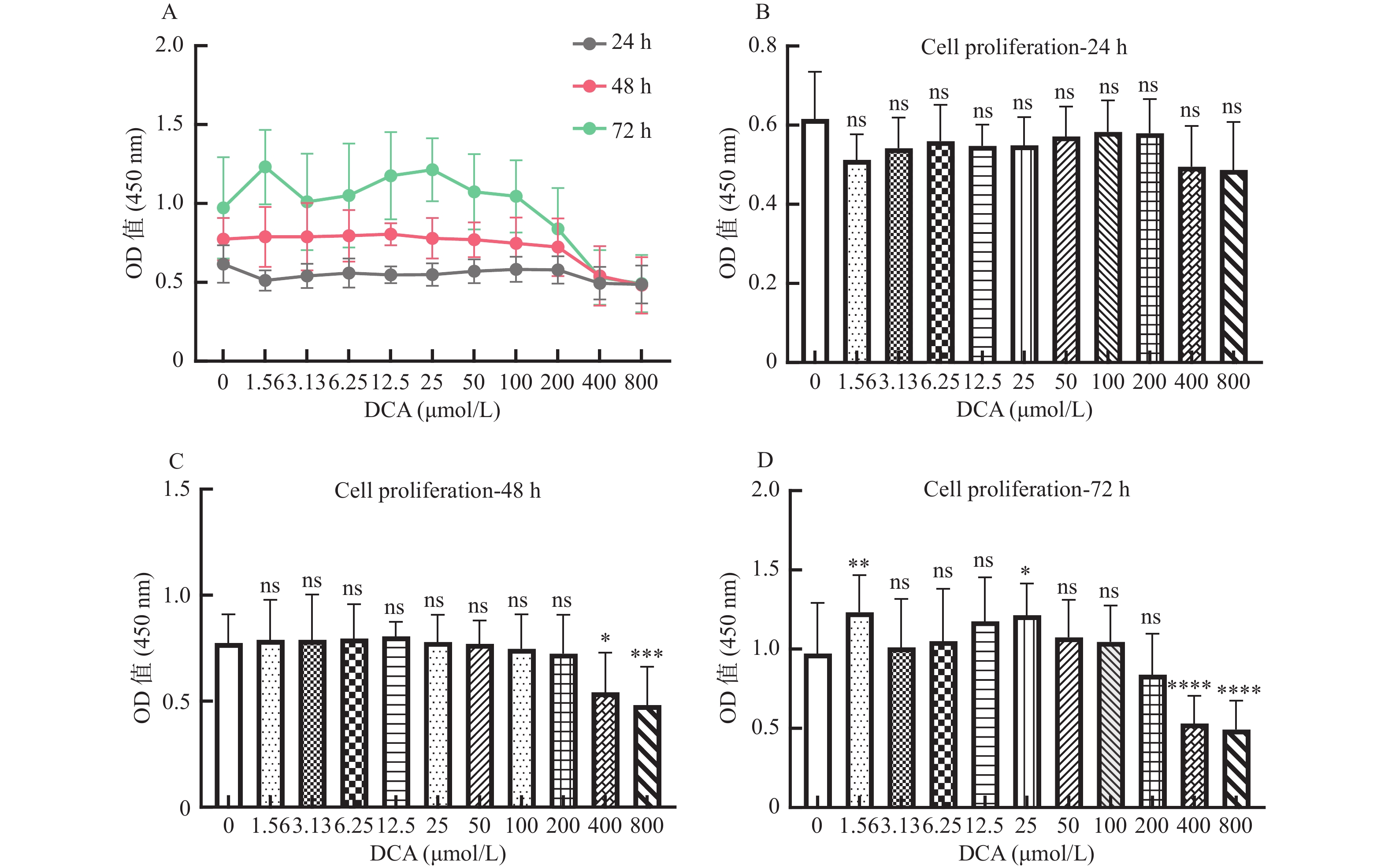
图 1 脱氧胆酸不同浓度及不同作用时间对 hUC-MSCs 增殖的影响
A:脱氧胆酸不同浓度及不同作用时间对 hUC-MSCs 增殖的影响;B:不同浓度脱氧胆酸作用 24 h 时 hUC-MSCs增殖情况;C:不同浓度脱氧胆酸作用48 h时hUC-MSCs增殖情况;D:不同浓度脱氧胆酸作用72 h时hUC-MSCs 增殖情况。各浓度组与0.00 μmol/L浓度组比较,ns表示差异无统计学意义,*表示 P < 0.05,**表示 P < 0.01,***表示 P < 0.001,****表示 P < 0.0001。
Figure 1. Effects of DCA with different concentration and different time on the proliferation of hUC- MSCs
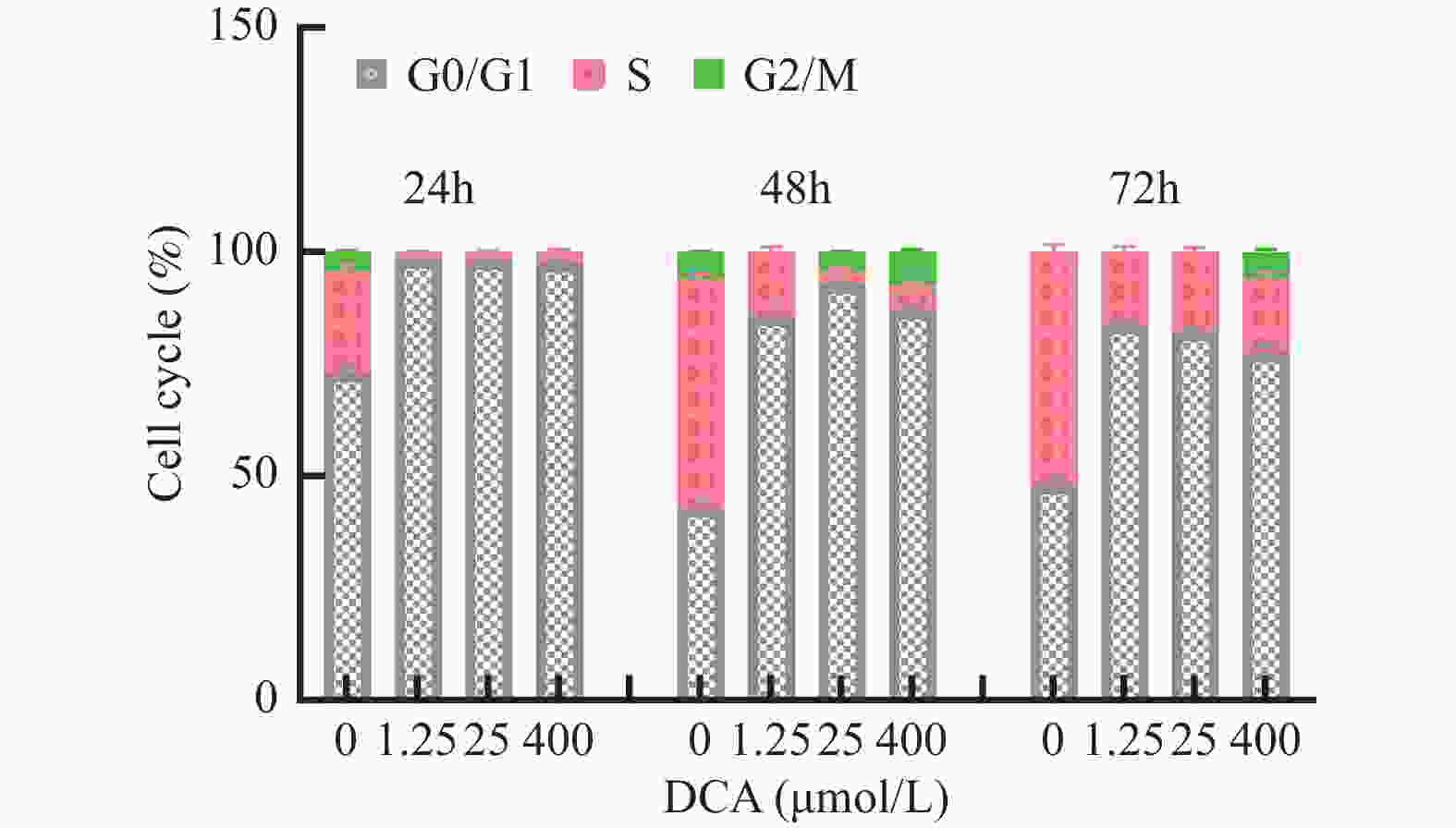
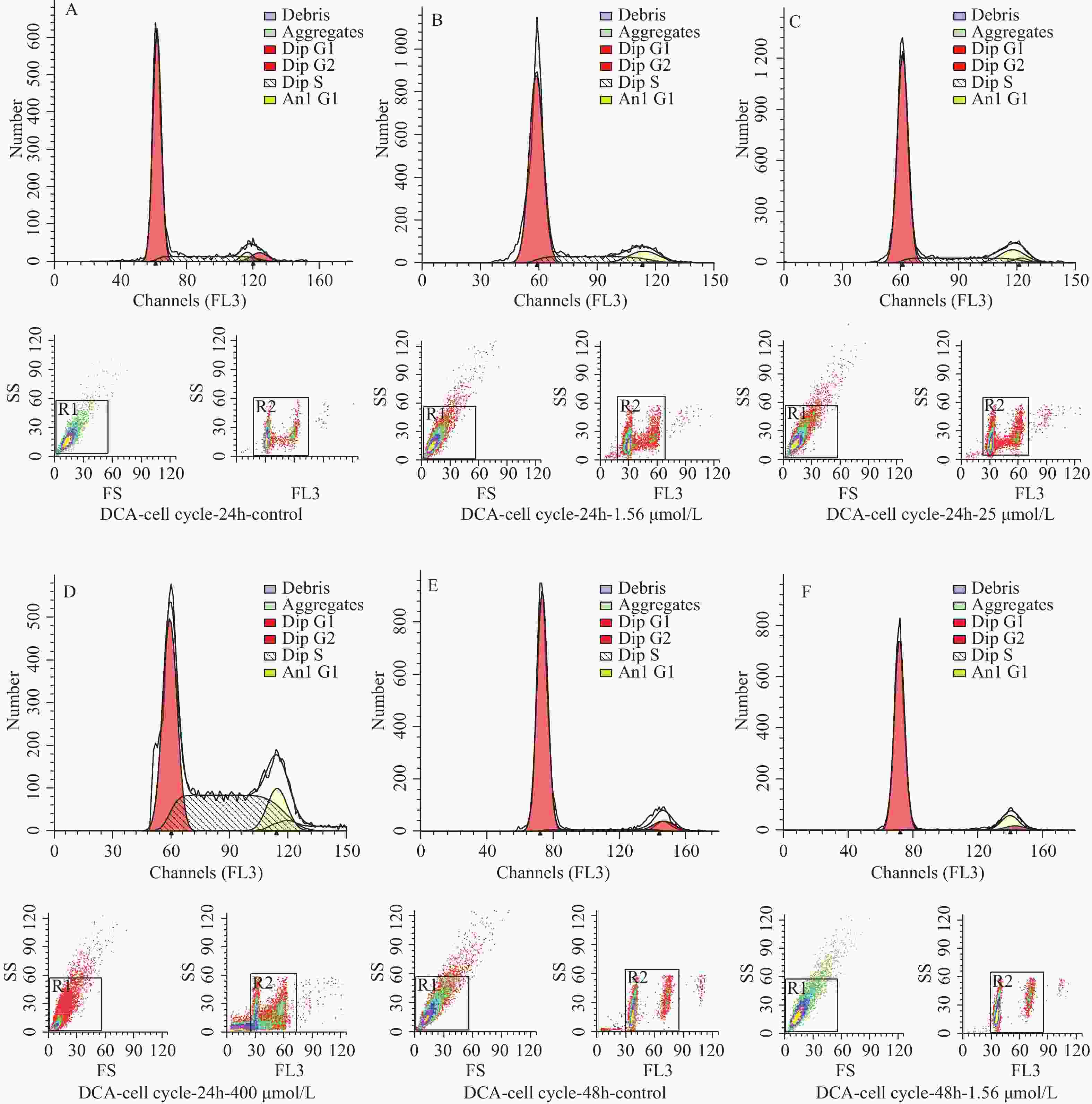
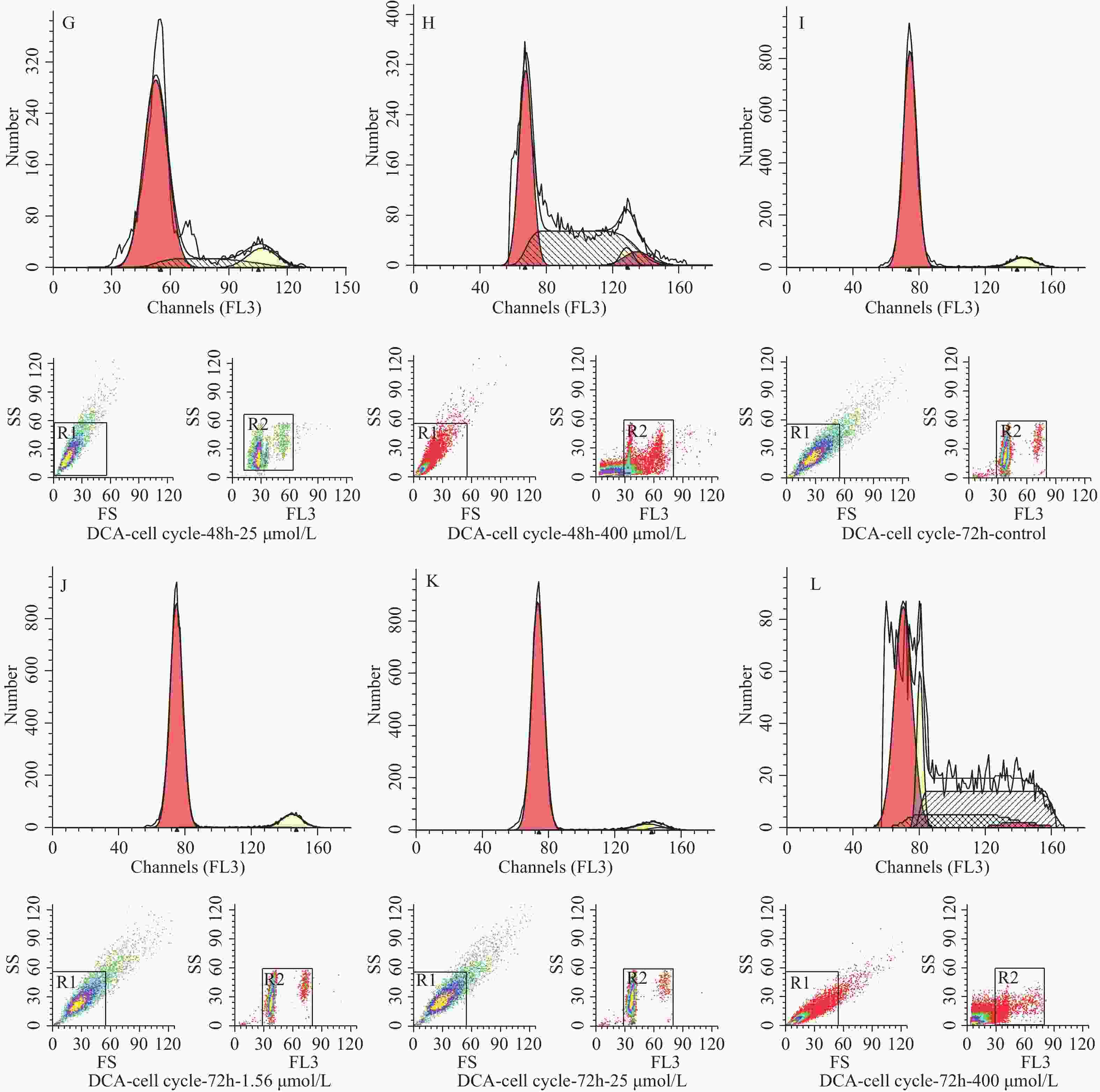
图 3 不同浓度及不同作用时间脱氧胆酸刺激后流式细胞技术检测 hUC-MSCs 细胞周期代表图例
A~D:24 h时脱氧胆酸0.00 μmol/L~400.00 μmol/L hUC-MSCs细胞周期分布情况;E~H:48 h时脱氧胆酸0.00 μmol/L~400.00 μmol/L hUC-MSCs细胞周期分布情况;I~L:72 h时脱氧胆酸0.00 μmol/L~400.00 μmol/L hUC-MSCs细胞周期分布情况。
Figure 3. Cell cycle of hUC-MSCs tested by flow cytometry after different concentration and different time of DCA
表 1 脱氧胆酸不同浓度及不同作用时间hUC-MSCs吸光度值(OD450nm值)(
$\bar x \pm s $ )Table 1. Absorbance (OD450nm) of hUC-MSCs at different dose and time of DCA (
$\bar x \pm s $ )脱氧胆酸浓度(μmol/L) 样本数 24 h 48 h 72 h 0.00(对照组) 45 0.61 ± 0.12 0.77 ± 0.14* 0.97 ± 0.32○* 1.56 45 0.51 ± 0.07 0.79 ± 0.19* 1.23 ± 0.24○* 3.13 45 0.54 ± 0.08 0.79 ± 0.21* 1.01 ± 0.31○* 6.25 45 0.56 ± 0.09 0.80 ± 0.16* 1.05 ± 0.33○* 12.50 45 0.55 ± 0.05 0.81 ± 0.07* 1.18 ± 0.28○* 25.00 45 0.55 ± 0.07 0.78 ± 0.13* 1.21 ± 0.20○* 50.00 45 0.57 ± 0.08 0.77 ± 0.03* 1.07 ± 0.24○* 100.00 45 0.58 ± 0.08 0.75 ± 0.16* 1.05 ± 0.23○* 200.00 44 0.58 ± 0.09 0.72 ± 0.16 0.84 ± 0.26* 400.00 45 0.50 ± 0.10 0.54 ± 0.19 0.53 ± 0.17 800.00 45 0.49 ± 0.12 0.48 ± 0.18 0.49 ± 0.18 与24 h比较,*P < 0.05;与48 h比较,○P < 0.05。 表 2 脱氧胆酸不同浓度及不同作用时间 hUC-MSCs 细胞周期比例(%)
Table 2. Cell cycle ratio of hUC-MSCs with different concentration and different time of DCA (%)
时间
(h)分期 0.00
μmol/L1.56
μmol/L25.00
μmol/L400.00
μmol/L24 G0/G1期 77.60 82.33* 83.77* 48.17* S期 16.48 17.67 16.23 51.83* G2/M期 5.91 0.00* 0.00* 0.00* 48 G0/G1期 87.07△ 92.85*△ 85.73*△ 42.99*△ S期 5.45△ 2.92*△ 14.27*△ 50.89* G2/M期 7.48△ 4.23*△ 0.00* 6.12*△ 72 G0/G1期 97.60△ 98.19*△ 98.41*△ 73.14*△ S期 2.40△ 1.81*△ 1.59*△ 22.70*△ G2/M期 0.00△ 0.00 0.00 4.16*△ 与0.00 µmol/L比较,*P < 0.0001;与24 h比较,△P < 0.0001。 -
[1] Ridlon J M,Kang D J,Hylemon P B. Bile salt biotransformations by human intestinal bacteria[J]. J Lipid Res,2006,47(2):241-259. doi: 10.1194/jlr.R500013-JLR200 [2] Huang D,Xiong M,Xu X,et al. Bile acids elevated by high-fat feeding induce endoplasmic reticulum stress in intestinal stem cells and contribute to mucosal barrier damage[J]. Biochem Biophys Res Commun,2020,529(2):289-295. doi: 10.1016/j.bbrc.2020.05.226 [3] Salim S Y,Soderholm J D. Importance of disrupted intestinal barrier in inflammatory bowel diseases[J]. Inflamm Bowel Dis,2011,17(1):362-381. doi: 10.1002/ibd.21403 [4] Wang Z,Litterio M C,Muller M,et al. (-)-Epicatechin and NADPH oxidase inhibitors prevent bile acid-induced Caco-2 monolayer permeabilization through ERK1/2 modulation[J]. Redox Biol,2020,28:101360. doi: 10.1016/j.redox.2019.101360 [5] Zeng H,Safratowhich B D,Cheng W H,et al. Deoxycholic acid modulates cell-junction gene expression and increases intestinal barrier dysfunction[J]. Molecules,2022,27(3):723. doi: 10.3390/molecules27030723 [6] Jiao Y,Sun Y T,Chen N F,et al. Human umbilical cord-derived mesenchymal stem cells promote repair of neonatal brain injury caused by hypoxia/ischemia in rats[J]. Neural Regen Res,2022,17(11):2518-2525. doi: 10.4103/1673-5374.339002 [7] Ma C,Feng Y,Yang L,et al. In vitro immunomodulatory effects of human umbilical cord-derived mesenchymal stem cells on peripheral blood cells from warm autoimmune hemolytic anemia patients[J]. Acta Haematol,2022,145(1):63-71. doi: 10.1159/000506759 [8] Chen Y,Hu Y,Zhou X,et al. Human umbilical cord-derived mesenchymal stem cells ameliorate psoriasis-like dermatitis by suppressing IL-17-producing gammadelta T cells[J]. Cell Tissue Res,2022,388(3):549-563. doi: 10.1007/s00441-022-03616-x [9] Guo G,Tan Z,Liu Y,et al. The therapeutic potential of stem cell-derived exosomes in the ulcerative colitis and colorectal cancer[J]. Stem Cell Res Ther,2022,13(1):138. doi: 10.1186/s13287-022-02811-5 [10] Li X,Wang Q,Ding L,et al. Intercellular adhesion molecule-1 enhances the therapeutic effects of MSCs in a dextran sulfate sodium-induced colitis models by promoting MSCs homing to murine colons and spleens[J]. Stem Cell Res Ther,2019,10(1):267. doi: 10.1186/s13287-019-1384-9 [11] Franzosa E A,Sirota-Madi A,Avila-Pacheco J,et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease[J]. Nat Microbiol,2019,4(2):293-305. [12] Appleby R N,Walters J R. The role of bile acids in functional GI disorders[J]. Neurogastroenterol Motil,2014,26(8):1057-1069. doi: 10.1111/nmo.12370 [13] Wahlstrom A,Sayin S I,Marschall H U,et al. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism[J]. Cell Metab,2016,24(1):41-50. doi: 10.1016/j.cmet.2016.05.005 [14] Kuenzig M E,Fung S G,Marderfeld L,et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease:Systematic review[J]. Gastroenterology,2022,162(4):1147-1159.e4. doi: 10.1053/j.gastro.2021.12.282 [15] Carroll M W,Kuenzig M E,Mack D R,et al. The impact of inflammatory bowel disease in Canada 2018:Children and adolescents with IBD[J]. J Can Assoc Gastroenterol,2019,2(Suppl 1):S49-S67. [16] Yaghooti H,Mohammadtaghvaei N,Mahboobnia K. Effects of palmitate and astaxanthin on cell viability and proinflammatory characteristics of mesenchymal stem cells[J]. Int Immunopharmacol,2019,68:164-170. doi: 10.1016/j.intimp.2018.12.063 [17] Lopez Perez R,Munz F,Vidoni D,et al. Mesenchymal stem cells preserve their stem cell traits after exposure to antimetabolite chemotherapy[J]. Stem Cell Res,2019,40:101536. doi: 10.1016/j.scr.2019.101536 [18] Zeng H,Claycombe K J,Reindl K M. Butyrate and deoxycholic acid play common and distinct roles in HCT116 human colon cell proliferation[J]. J Nutr Biochem,2015,26(10):1022-1028. doi: 10.1016/j.jnutbio.2015.04.007 [19] 吴二斌,杨海波,鲁亚芳,等. 脱氧胆酸对人胃癌BGC-823细胞凋亡的作用[J]. 广东医学,2016,37(12):1795-1798. doi: 10.13820/j.cnki.gdyx.2016.12.004 [20] Gandola Y B,Fontana C,Bojorge M A,et al. Concentration-dependent effects of sodium cholate and deoxycholate bile salts on breast cancer cells proliferation and survival[J]. Mol Biol Rep,2020,47(5):3521-3539. doi: 10.1007/s11033-020-05442-2 [21] Zhang G,Zhang J,Shang D,et al. Deoxycholic acid inhibited proliferation and induced apoptosis and necrosis by regulating the activity of transcription factors in rat pancreatic acinar cell line AR42J[J]. In Vitro Cell Dev Biol Anim,2015,51(8):851-856. doi: 10.1007/s11626-015-9907-x [22] Wang L,Gong Z,Zhang X,et al. Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation[J]. Gut Microbes,2020,12(1):1-20. [23] Edelstein A,Fink D,Musch M,et al. Protective effects of nonionic triblock copolymers on bile acid-mediated epithelial barrier disruption[J]. Shock,2011,36(5):451-457. doi: 10.1097/SHK.0b013e31822d8de1 [24] Jian J,Nie M T,Xiang B,et al. Rifaximin ameliorates non-alcoholic steatohepatitis in mice through regulating gut microbiome-related bile acids[J]. Frontiers in Pharmacology,2022,13:841132. doi: 10.3389/fphar.2022.841132 [25] Yang M,Gu Y,Li L,et al. Bile acid-gut microbiota axis in inflammatory bowel disease:From bench to bedside[J]. Nutrients,2021,13(9):3143. doi: 10.3390/nu13093143 [26] Mens M M J,Ghanbari M. Cell cycle regulation of stem cells by MicroRNAs[J]. Stem Cell Rev Rep,2018,14(3):309-322. doi: 10.1007/s12015-018-9808-y [27] Yang H B,Song W,Cheng M D,et al. Deoxycholic acid inhibits the growth of BGC-823 gastric carcinoma cells via a p53 mediated pathway[J]. Mol Med Rep,2015,11(4):2749-2754. doi: 10.3892/mmr.2014.3004 [28] Barta T,Dolezalova D,Holubcova Z,et al. Cell cycle regulation in human embryonic stem cells: Links to adaptation to cell culture[J]. Exp Biol Med (Maywood),2013,238(3):271-275. doi: 10.1177/1535370213480711 -






 下载:
下载: