The Levels of Serum CXCL5,TLR4,and MMP-3 in Gouty Arthritis Patients with Different Clinical Features and Their Correlation with the Joint Destruction and Serum Uric Acid Levels
-
摘要:
目的 分析不同临床特征痛风性关节炎(GA)患者血清趋化因子5(CXCL5)、Toll样受体4(TLR4)、基质金属蛋白酶-3(MMP-3)水平与关节破坏程度、血尿酸(SUA)水平的相关性及其重度GA预测价值。 方法 选择2023年6月至2025年6月期间于西安市人民医院确诊的212例GA患者(GA组)展开研究,再纳入212例体检健康者(对照组)与GA组进行对照,受试者工作特征(ROC)曲线进行预测价值分析;Pearson法进行相关性分析。 结果 GA组CXCL5、TLR4、MMP-3水平均高于对照组(P < 0.05)。不同临床分期、受累关节、痛风石、病程及年发作频率的GA患者血清CXCL5、TLR4、MMP-3表达水平比较差异有统计学意义(P < 0.05)。重度GA组CXCL5、TLR4、MMP-3水平均高于轻度GA组(P < 0.05)。当血清CXCL5、TLR4、MMP-3分别水平>612.56 pg/mL、11.15 ng/mL、12.06 ng/mL时,GA患者重度的风险更高。CXCL5、TLR4、MMP-3联合对重度GA预测的曲线下面积(AUC)为0.963。与低表达者相比,CXCL5、TLR4、MMP-3高表达者重度GA的风险分别增高2.758、2.184、2.227倍。CXCL5、TLR4、MMP-3是病情的影响因素(P < 0.05)。与低SUA组相比,高SUA组血清CXCL5、TLR4、MMP-3水平均更高(P < 0.05)。 结论 GA患者血清CXCL5、TLR4、MMP-3高表达,且与GA患者临床特征、关节破坏程度及SUA水平显著相关,临床价值较高。 Abstract:Objective To analyze the correlation between serum levels of C-X-C motif chemokine 5 (CXCL5), Toll-like receptor 4 (TLR4), and matrix metalloproteinase-3 (MMP-3) with the degree of joint destruction and serum uric acid (SUA) levels in gouty arthritis patients with different clinical characteristics, and to evaluate their predictive value for severe GA. Methods A total of 212 GA patients (GA group) diagnosed at Xi'an People's Hospital between June 2023 and June 2025 were enrolled, along with 212 healthy individuals (control group). Predictive value was assessed using receiver operating characteristic (ROC) curve analysis, and correlation was assessed using Pearson method. Results Serum levels of CXCL5, TLR4, and MMP-3 were significantly higher in the GA group compared to the control group (P < 0.05). Statistically significant differences in serum levels of CXCL5, TLR4, and MMP-3 were observed among GA patients with different clinical stages, affected joints, presence of tophi, disease duration, and annual attack frequency (P < 0.05). The severe GA group had higher levels of CXCL5, TLR4, and MMP-3 compared to the mild GA group (P < 0.05). GA patients were at higher risk for severe disease when serum CXCL5, TLR4, and MMP-3 levels exceeded 612.56 pg/mL, 11.15 ng/mL, and 12.06 ng/mL, respectively. The combined prediction of severe GA by CXCL5, TLR4, and MMP-3 yielded an area under the curve (AUC) of 0.963. Compared to low-expression patients, those with high expression of CXCL5, TLR4, and MMP-3 had a 2.758-fold, 2.184-fold, and 2.227-fold increased risk of severe GA, respectively. CXCL5, TLR4, and MMP-3 were identified as influencing factors for the disease severity (P < 0.05). The high SUA group had significantly higher serum levels of CXCL5, TLR4, and MMP-3 compared to the low SUA group (P < 0.05). Conclusion GA patients exhibit high serum expression of CXCL5, TLR4, and MMP-3, which are significantly associated with clinical features, the degree of joint destruction, and SUA levels, demonstrating considerable clinical value. -
表 1 对照组与GA组一般资料及CXCL5、TLR4、MMP-3比较[($ \bar{x} \pm s $)/n(%)]
Table 1. Comparison of general data and serum CXCL5,TLR4,and MMP-3 levels between control group and GA group[($ \bar{x} \pm s $)/n(%)]
指标 对照组(n=212) GA组(n=212) χ2/t P 性别 男 109(51.42) 116(54.72) 0.464 0.496 女 103(48.58) 96(45.28) 年龄(岁) 46.31 ± 9.14 45.67 ± 8.26 0.756 0.450 CXCL5(pg/mL) 295.63 ± 71.45 611.80 ± 145.72 28.365 <0.001* TLR4(ng/mL) 3.25 ± 0.62 11.35 ± 2.18 52.036 <0.001* MMP-3(ng/mL) 5.73 ± 1.16 12.93 ± 2.93 33.267 <0.001* *P < 0.05;各组数据均经Shapiro-Wilk正态性检验及方差齐性检验,正态分布且方差齐(P > 0.05)。 表 2 不同临床特征GA患者CXCL5、TLR4、MMP-3比较[M(Q1,Q3)/($ \bar{x} \pm s $)]
Table 2. Comparison of serum CXCL5,TLR4,and MMP-3 levels among GA patients with different clinical characteristics [M (Q1,Q3)/($ \bar{x} \pm s $)]
特征 n CXCL5(pg/mL) H/F/t P TLR4(ng/mL) H/F/t P MMP-3(ng/mL) H/F/t P 临床分期 急性期 139 691.36(581.45,813.62)ab 75.621 <0.001* 12.95(9.86,17.25)ab 189.354 <0.001* 14.92(11.07,20.42)ab 107.825 <0.001* 慢性期 43 535.89(465.82,608.92)a 8.06(6.37,11.26)a 9.65(7.43,14.91)a 间歇期 30 335.63(281.42,379.65) 3.95(1.14,6.07) 6.53(4.08,9.13) 受累关节 ≥15个 80 741.53 ± 176.24 10.418 <0.001* 13.92 ± 2.62 13.486 <0.001* 14.83 ± 3.56 7.837 <0.001* <15个 132 533.18 ± 114.91 9.79 ± 1.83 11.78 ± 2.11 痛风石 有 63 752.64 ± 183.29 9.828 <0.001* 14.08 ± 2.74 11.096 <0.001* 15.19 ± 3.74 7.723 <0.001* 无 149 552.25 ± 109.75 10.20 ± 2.13 11.97 ± 2.25 病程 ≥5年 78 738.14 ± 171.46 10.188 <0.001* 14.26 ± 2.72 14.761 <0.001* 15.31 ± 3.61 9.654 <0.001* <5年 134 538.26 ± 113.76 9.66 ± 1.81 11.54 ± 2.08 年发作频率 ≥3次 95 802.31 ± 193.62 16.395 <0.001* 15.13 ± 2.86 21.554 <0.001* 14.28 ± 3.72 6.176 <0.001* <3次 117 457.11 ± 108.17 8.28 ± 1.72 11.83 ± 1.93 *P < 0.05;与间歇期比较,aP < 0.05;与慢性期比较,bP < 0.05;各组数据均经Shapiro-Wilk正态性检验及方差齐性检验:间歇期组CXCL5、TLR4、MMP-3的P值分别为0.023、0.015、0.038,进行非参数检验;其他指标均符合正态分布,方差齐(P > 0.05)。 表 3 不同关节破坏程度患者指标比较($ \bar{x} \pm s $)
Table 3. Comparison of indicators among patients with different degrees of joint destruction($ \bar{x} \pm s $)
指标 重度GA组(n=119) 轻中度GA组(n=93) t P CXCL5(pg/mL) 756.34 ± 214.73 426.85 ± 113.65 13.399 <0.001* TLR4(ng/mL) 14.63 ± 3.25 7.15 ± 1.69 20.160 <0.001* MMP-3(ng/mL) 15.09 ± 3.62 10.16 ± 2.17 11.602 <0.001* β-CTX(ng/mL) 1.45 ± 0.23 1.10 ± 0.18 12.067 <0.001* RANKL(pg/mL) 123.46 ± 48.71 178.52 ± 56.19 7.633 <0.001* TRACP5b(ng/mL) 3.85 ± 1.06 2.76 ± 0.64 8.746 <0.001* sICAM-1(pg/mL) 175.63 ± 30.45 114.28 ± 13.86 18.019 <0.001* sVCAM-1(pg/mL) 214.96 ± 35.62 134.18 ± 15.21 20.453 <0.001* *P < 0.05;均符合正态分布,方差齐(P > 0.05)。 表 4 CXCL5、TLR4、MMP-3预测价值
Table 4. Predictive value of CXCL5,TLR4 and MMP-3
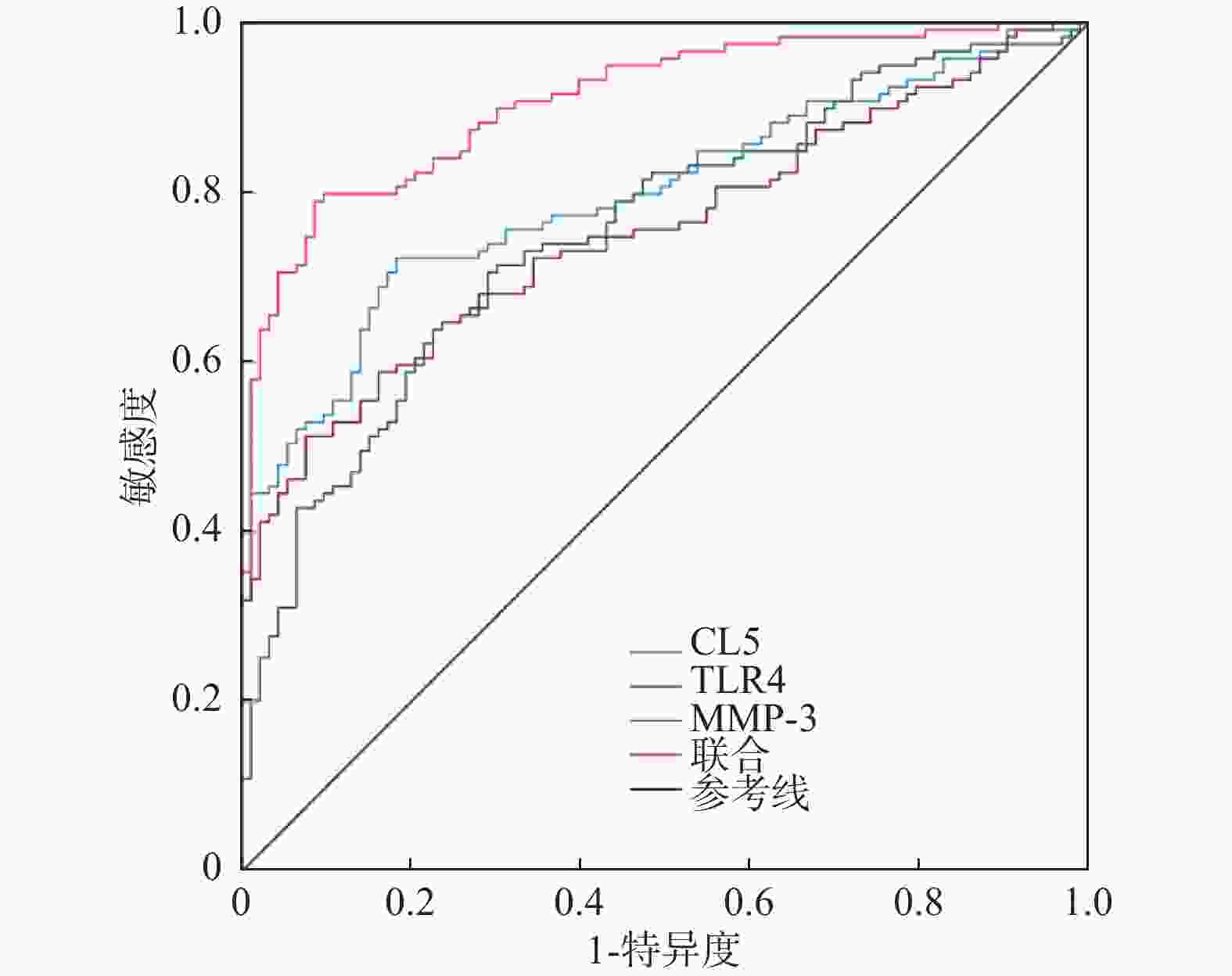
预测项目 AUC(95%CI) 敏感度(95%CI,%) 特异度(95%CI,%) 约登指数 界值 CXCL5 0.797(0.737~0.849) 72.27(63.30~80.10) 81.72(72.40~89.00) 0.540 623.51 TLR4 0.755(0.691~0.811) 70.59(61.50~78.60) 70.97(60.60~79.90) 0.416 10.49 MMP-3 0.754(0.690~0.810) 51.26(41.90~60.50) 92.47(85.10~96.90) 0.437 12.14 联合 0.963(0.927~0.984) 86.55(79.80~92.40) 96.77(90.30~99.30) 0.833 ~ 表 5 高、低表达CXCL5、TLR4、MMP-3与重度GA的关系[n(%)]
Table 5. Relationship between high and low expression of CXCL5,TLR4,MMP-3 and severe GA[n(%)]
指标 重度GA组(n=119) 轻中度GA组(n=93) 相对危险度(95%CI) χ2 P CXCL5 高表达 86(72.27) 17(18.28) 2.758(2.048~3.714) 60.914 <0.001* 低表达 33(27.73) 76(81.72) TLR4 高表达 84(70.59) 27(29.03) 2.184(1.638~2.912) 36.140 <0.001* 低表达 35(29.41) 66(70.97) MMP-3 高表达 61(51.26) 7(7.53) 2.227(1.797~2.760) 45.827 <0.001* 低表达 58(48.74) 86(92.47) *P < 0.05;均符合正态分布,方差齐(P > 0.05)。 表 6 病情影响因素分析
Table 6. Analysis of factors influencing disease severity
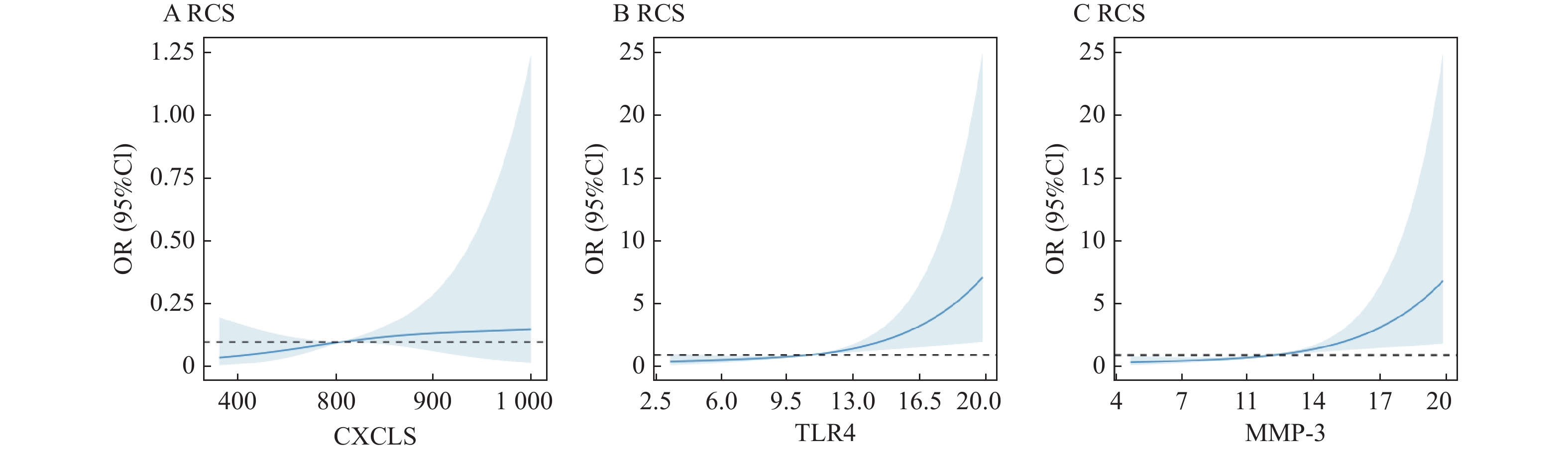
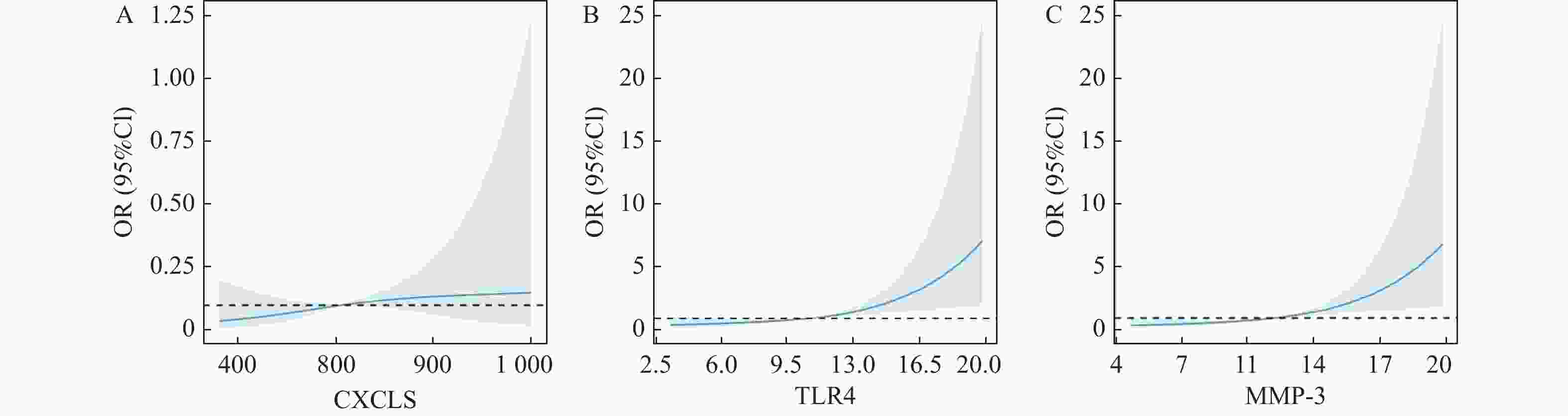
影响因素 B SE Wald P OR 95%CI CXCL5 0.757 0.126 36.101 <0.001* 2.132 1.665~2.729 TLR4 0.680 0.136 25.005 <0.001* 1.974 1.512~2.577 MMP-3 0.791 0.218 13.172 <0.001* 2.206 1.439~3.382 *P < 0.05。 表 7 不同SUA水平患者的匹配[($\bar x \pm s $)/n(%)]
Table 7. Matching of patients with different SUA levels[($\bar x \pm s$)/n(%)]
资料 高SUA组
(n=135/50)低SUA组
(n=77/50)SMD 年龄(岁) 匹配前 45.08 ± 8.12 46.70 ± 8.57 0.315 匹配后 45.16 ± 8.16 45.37 ± 8.39 0.021* 性别(男/女) 匹配前 73/62 43/34 0.136 匹配后 28/22 28/22 <0.001* *P < 0.05;均符合正态分布,方差齐(P > 0.05)。 表 8 匹配前不同SUA水平患者血清CXCL5、TLR4、MMP-3比较($\bar x \pm s $)
Table 8. Comparison of serum CXCL5,TLR4 and MMP-3 levels among patients with different SUA levels before matching ($\bar x \pm s $)
组别 n CXCL5(pg/mL) TLR4(ng/mL) MMP-3(ng/mL) 高SUA组 135 716.25 ± 203.68 13.26 ± 2.87 14.56 ± 3.24 低SUA组 77 428.67 ± 127.45 8.00 ± 1.36 10.07 ± 2.13 t − 11.196 15.131 10.887 P − <0.001* <0.001* <0.001* *P < 0.05。 表 9 匹配后不同SUA水平患者血清CXCL5、TLR4、MMP-3比较($\bar x \pm s$)
Table 9. Comparison of serum CXCL5,TLR4 and MMP-3 levels among patients with different SUA levels after matching ($\bar x \pm s $)
组别 n CXCL5(pg/mL) TLR4(ng/mL) MMP-3(ng/mL) 高SUA组 50 725.63 ± 211.47 14.07 ± 2.91 14.69 ± 3.15 低SUA组 50 421.75 ± 120.68 8.04 ± 1.34 10.02 ± 2.08 t − 8.825 13.309 8.748 P − <0.001* <0.001* <0.001* *P < 0.05;匹配前、后高SUA组与低SUA组各指标均符合正态分布,方差齐(P > 0.05)。 表 10 相关性分析
Table 10. Correlation analysis
指标 CXCL5 TLR4 MMP-3 r P 验证P r P 验证P r P 验证P β-CTX 0.354 <0.001* 0.034* 0.406 <0.001* 0.031* 0.425 <0.001* 0.029* RANKL −0.462 <0.001* 0.021* −0.513 <0.001* 0.013* −0.631 <0.001* 0.010* TRACP5b 0.681 <0.001* 0.005* 0.391 <0.001* 0.032* 0.557 <0.001* 0.011* sICAM-1 0.661 <0.001* 0.009* 0.426 <0.001* 0.025* 0.426 <0.001* 0.027* sVCAM-1 0.529 <0.001* 0.011* 0.671 <0.001* 0.007* 0.369 <0.001* 0.030* SUA 0.461 <0.001* 0.022* 0.506 <0.001* 0.015* 0.478 <0.001* 0.019* *P < 0.05;各组数据符合正态分布,方差齐(P > 0.05),满足相关性分析条件;对r值,使用t检验统计量,验证*P均<0.05,拒绝原假设(即变量间无线性相关关系),存在显著相关性。 -
[1] Yao T K, Lee R P, Wu W T, et al. Advances in gouty arthritis management: Integration of established therapies, emerging treatments, and lifestyle interventions[J]. Int J Mol Sci, 2024, 25(19): 10853. doi: 10.3390/ijms251910853 [2] Yin C, Lyu Q, Dong Z, et al. Well-defined alginate oligosaccharides ameliorate joint pain and inflammation in a mouse model of gouty arthritis[J]. Theranostics, 2024, 14(8): 3082-3103. doi: 10.7150/thno.95611 [3] Hou Z, Ma A, Mao J, et al. Overview of the pharmacokinetics and pharmacodynamics of URAT1 inhibitors for the treatment of hyperuricemia and gout[J]. Expert Opin Drug Metab Toxicol, 2023, 19(12): 895-909. doi: 10.1080/17425255.2023.2287477 [4] Chang T T, Li S Y, Tsai M T, et al. CXCL5 inhibition ameliorates acute kidney injury and prevents the progression from acute kidney injury to chronic kidney disease[J]. Clin Sci (Lond), 2024, 138(22): 1451-1466. doi: 10.1042/CS20241713 [5] Brandolini L, Aramini A, Bianchini G, et al. Inflammation-independent antinociceptive effects of DF2755A, a CXCR1/2 selective inhibitor: A new potential therapeutic treatment for peripheral neuropathy associated to non-ulcerative interstitial cystitis/bladder pain syndrome[J]. Front Pharmacol, 2022, 13: 854238. doi: 10.3389/fphar.2022.854238 [6] Ciesielska A, Matyjek M, Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling[J]. Cell Mol Life Sci, 2021, 78(4): 1233-1261. doi: 10.1007/s00018-020-03656-y [7] Shi B, Guo X, Iv A, et al. Polymorphism of MMP-3 gene and imbalance expression of MMP-3/TIMP-1 in articular cartilage are associated with an endemic osteochondropathy, Kashin- Beck disease[J]. BMC Musculoskelet Disord, 2022, 23(1): 3. doi: 10.1186/s12891-021-04952-9 [8] 中华医学会, 中华医学会杂志社, 中华医学会全科医学分会, 等. 痛风及高尿酸血症基层诊疗指南(2019年)[J]. 中华全科医师杂志, 2020, 19(4): 293-303. [9] 邵苗, 张学武. 2015年欧洲抗风湿病联盟/美国风湿病学会痛风分类新标准[J]. 中华风湿病学杂志, 2015, 19(12): 854-855 [10] van der Heijde D. How to read radiographs according to the sharp/van der heijde method[J]. J Rheumatol, 2000, 27(1): 261-263. [11] Dalbeth N, Doyle A J. Imaging tools to measure treatment response in gout[J]. Rheumatology (Oxford), 2018, 57(suppl 1): i27-i34. [12] Keller S F, Mandell B F. Management and cure of gouty arthritis[J]. Med Clin North Am, 2021, 105(2): 297-310. doi: 10.1016/j.mcna.2020.09.013 [13] Metzemaekers M, Mortier A, Vacchini A, et al. Endogenous modification of the chemoattractant CXCL5 alters receptor usage and enhances its activity toward neutrophils and monocytes[J]. Sci Signal, 2021, 14(673): eaax3053. doi: 10.1126/scisignal.aax3053 [14] Park S E, Park K, Kim E, et al. CXCL5/CXCL8 induces neutrophilic inflammation in peri-implantitis[J]. J Periodontal Res, 2024, 59(4): 698-711. doi: 10.1111/jre.13230 [15] Liang Y, Li H, Li J, et al. Role of neutrophil chemoattractant CXCL5 in SARS-CoV-2 infection-induced lung inflammatory innate immune response in an in vivo hACE2 transfection mouse model[J]. Zool Res, 2020, 41(6): 621-631. doi: 10.24272/j.issn.2095-8137.2020.118 [16] Li C, Huang Y, Wu C, et al. Astilbin inhibited neutrophil extracellular traps in gouty arthritis through suppression of purinergic P2Y6 receptor[J]. Phytomedicine, 2024, 130: 155754. doi: 10.1016/j.phymed.2024.155754 [17] Yin C, Liu B, Dong Z, et al. CXCL5 activates CXCR2 in nociceptive sensory neurons to drive joint pain and inflammation in experimental gouty arthritis[J]. Nat Commun, 2024, 15(1): 3263. doi: 10.1038/s41467-024-47640-7 [18] 冼英, 符洪明. 痛风性关节炎患者血清同型半胱氨酸、补体C3水平及其与尿酸的相关性[J]. 中外医学研究, 2023, 21(22): 71-73. [19] Silva C R, Saraiva A L, Rossato M F, et al. What do we know about toll-like receptors involvement in gout arthritis?[J]. Endocr Metab Immune Disord Drug Targets, 2023, 23(4): 446-457. doi: 10.2174/1871530322666220523145728 [20] Liu L, He S, Jia L, et al. Correlation analysis of serum TLR4 protein levels and TLR4 gene polymorphisms in gouty arthritis patients[J]. PLoS One, 2024, 19(4): e0300582. doi: 10.1371/journal.pone.0300582 [21] Lin S K, Chen S T, Zhan Y, et al. The alleviatory effects of koumine on MSU-induced gouty arthritis via the TLR4/NF-κB/NLRP3 pathway[J]. Basic Clin Pharmacol Toxicol, 2024, 135(2): 133-147. doi: 10.1111/bcpt.14037 [22] Li Z, Yu Y, Sun Q, et al. Based on the TLR4/NLRP3 pathway and its impact on the formation of NETs to explore the mechanism of ginsenoside Rg1 on acute gouty arthritis[J]. Int J Mol Sci, 2025, 26(9): 4233. doi: 10.3390/ijms26094233 [23] Jeong J, Lim M K, Han E H, et al. Extract of Aster glehni ameliorates potassium oxonate-induced hyperuricemia by modulating renal urate transporters and renal inflammation by suppressing TLR4/MyD88 signaling[J]. Food Sci Biotechnol, 2022, 31(13): 1729-1739. doi: 10.1007/s10068-022-01153-5 [24] Ruan H, Zhu T, Wang T, et al. Quercetin modulates ferroptosis via the SIRT1/nrf-2/HO-1 pathway and attenuates cartilage destruction in an osteoarthritis rat model[J]. Int J Mol Sci, 2024, 25(13): 7461. doi: 10.3390/ijms25137461 [25] Qin Y Y, Qin Z Y, Liang T, et al. Phellodendrine chloride alleviates gouty arthritis through IL-6/STAT3 signaling pathway[J]. Immunobiology, 2025, 230(4): 152918. doi: 10.1016/j.imbio.2025.152918 [26] Shi L, Yuan Z, Liu J, et al. Modified Simiaowan prevents articular cartilage injury in experimental gouty arthritis by negative regulation of STAT3 pathway[J]. J Ethnopharmacol, 2021, 270: 113825. doi: 10.1016/j.jep.2021.113825 [27] Zhuang H, Ren X, Jiang F, et al. Indole-3-propionic acid alleviates chondrocytes inflammation and osteoarthritis via the AhR/NF-κB axis[J]. Mol Med, 2023, 29(1): 17. doi: 10.1186/s10020-023-00614-9 [28] Wu Y, Wang Y, Yuan W, et al. Changes in serum inflammatory factors in acute gouty arthritis patients treated using ultrashort wave combined with loxoprofen sodium[J]. Pak J Med Sci, 2021, 37(7): 1788-1794. [29] Shi L, Liang T, Yang F, et al. Matrix Metalloproteinase-3 induces proteoglycan degradation in gouty arthritis model[J]. Gene, 2021, 765: 145120. doi: 10.1016/j.gene.2020.145120 [30] Bourmaud M, Zarka M, Le Cozannet R, et al. Effect of Rubus idaeus extracts in murine chondrocytes and explants[J]. Biomolecules, 2021, 11(2): 245. doi: 10.3390/biom11020245 -





 下载:
下载: