Cardiac Protective Mechanism of Dexmedetomidine and Its Application in Clinic
-
摘要: 心血管疾病仍然是围术期内导致患者死亡以及影响转归的重要因素。右美托咪定是α2肾上腺素受体激动剂,具有镇痛、镇静、抗焦虑等功能。总结右美托咪定在心肌缺血/再灌注损伤中不同种类的发现,并对其结果进行了相应机制的深入探讨。意在进一步阐明心脏疾病患者右美托咪定应用的潜在护心作用。Abstract: Cardiovascular disease remains a significant factor leading to patient mortality and influencing outcomes during the perioperative period. Dexmedetomidine is an α2-adrenergic receptor agonist with functions such as analgesia, sedation, and anti-anxiety. This review summarizes various types of findings regarding dexmedetomidine in myocardial ischemia/reperfusion injury and conducts in-depth discussions on the corresponding mechanisms of the results, aiming to further clarify the potential cardioprotective effects of dexmedetomidine in patients with heart diseases.
-
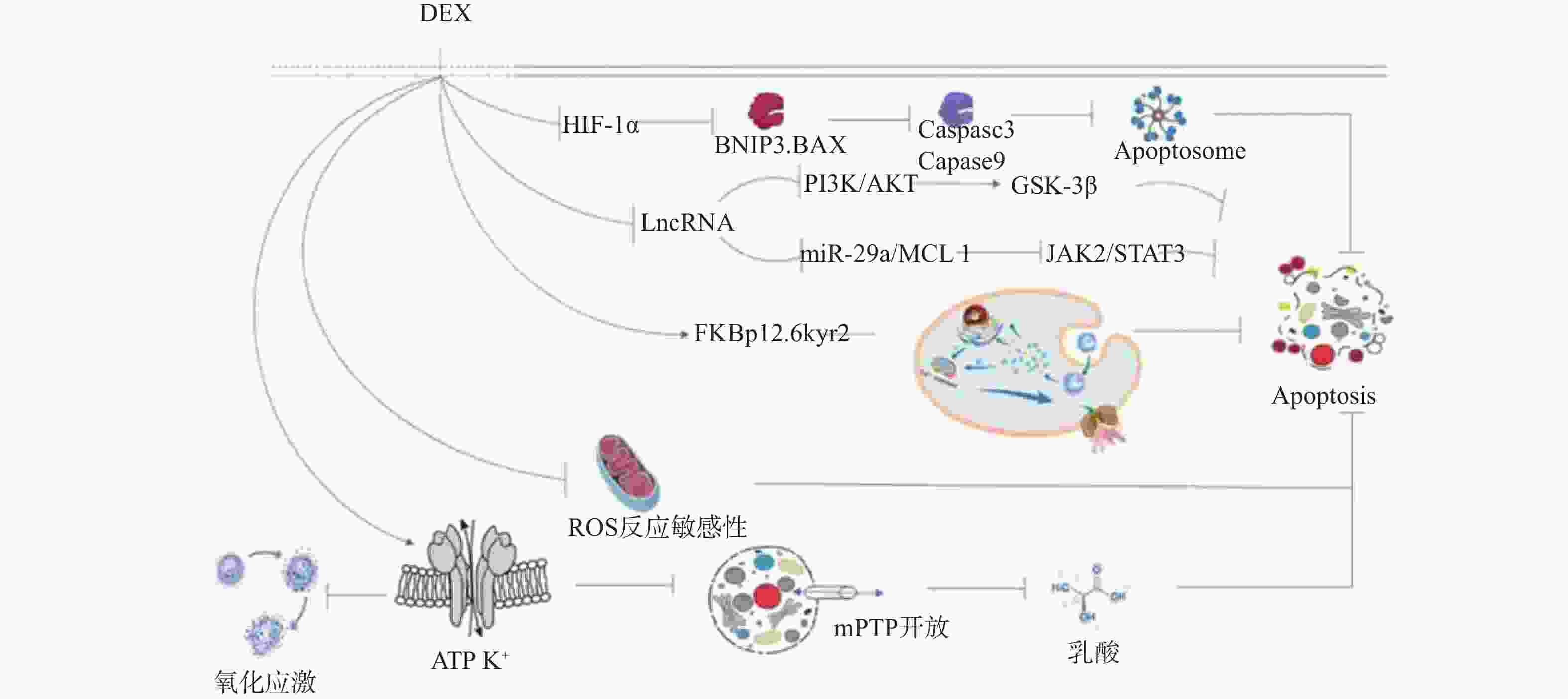
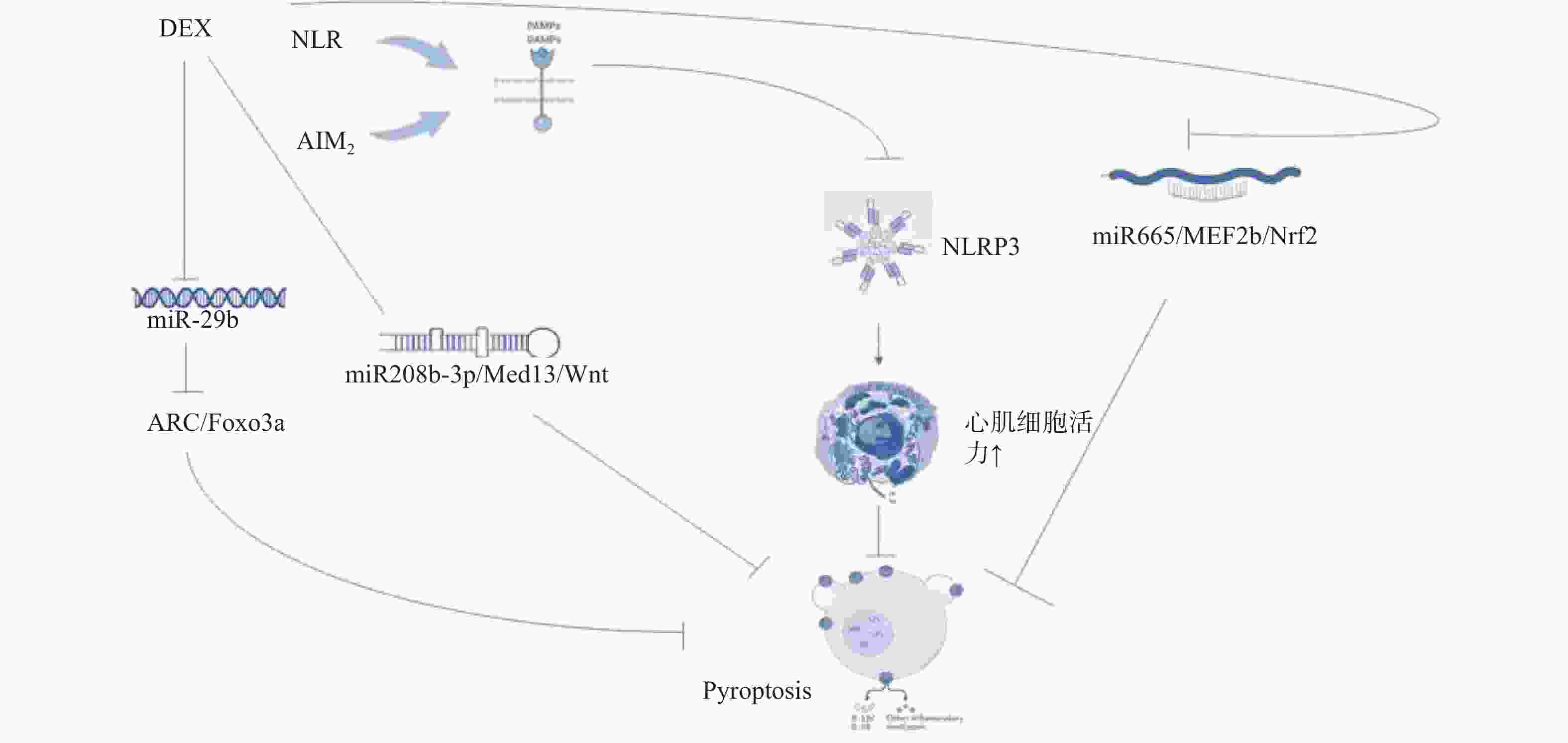
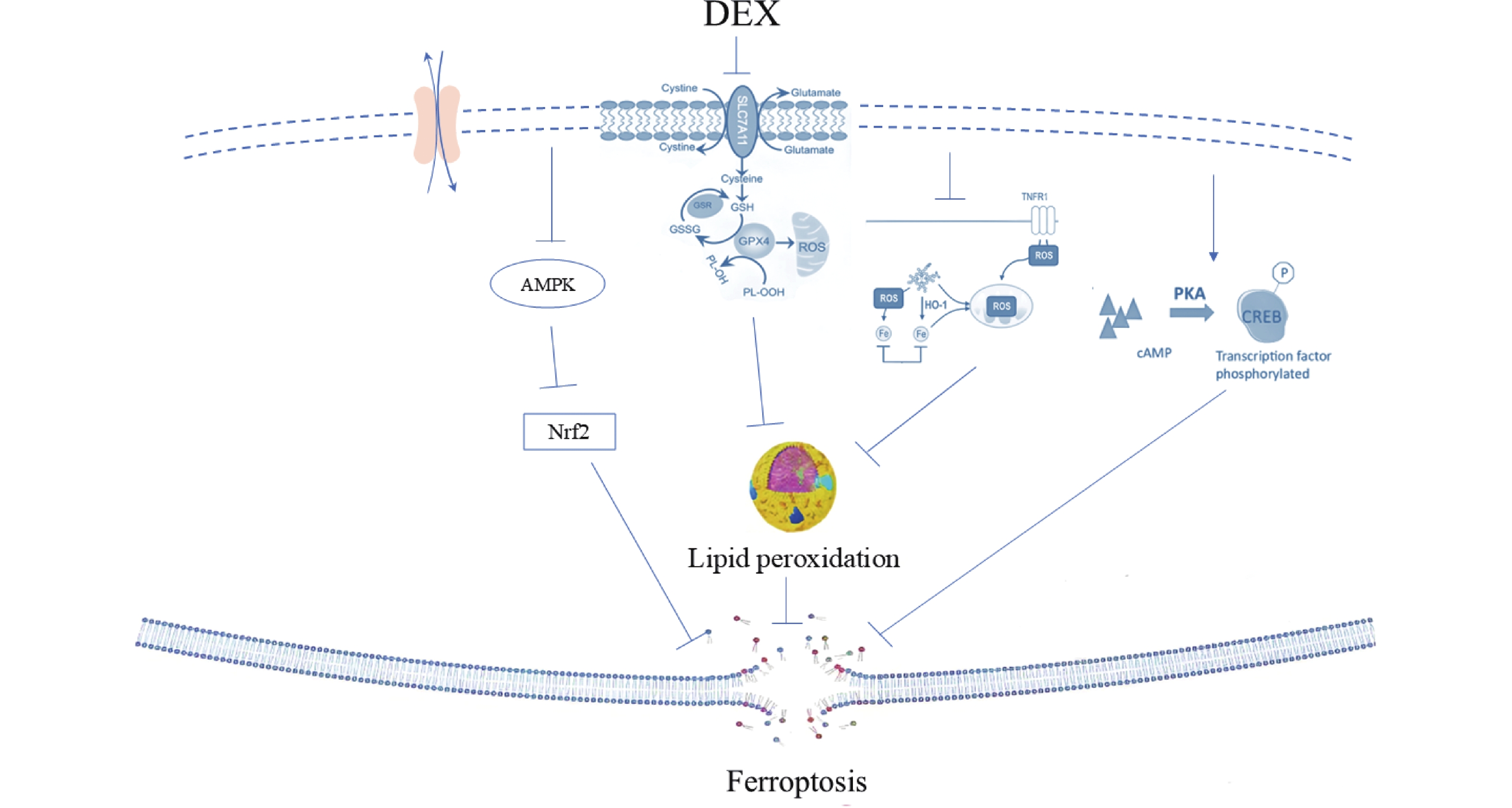
图 2 右美托咪定的凋亡内外途径机制图
DEX:右美托咪定;ATP:三磷酸腺苷;mPTP:线粒体通透性转换孔;ROS:活性氧;FKBp12.6/kyr2:fk506结合蛋白12.6/ryanodine受体2;Ca²+overload:钙离子超载;LncRNA:长链非编码RNA;JAK2/STAT3:蛋白质酪氨酸激酶2/信号转导与转录因子3;PI3K:磷脂酰肌醇-3激酶;AKT:蛋白激酶;Gsk-3β:糖原合成酶激酶-3β;HIF-1a:缺氧诱导因子-1a;BAX:相关X蛋白;Caspase3、Caspase9:半胱氨酸天冬氨酸蛋白酶3、9;Apoptosome:凋亡复合体;Apoptosis:凋亡。
Figure 2. Diagram of the internal and external pathways of apoptosis of dexmedetomidine
-
[1] He L, Hao S, Wang Y, et al. DEXmedetomidine preconditioning attenuates ischemia/reperfusion injury in isolated rat hearts with endothelial dysfunction[J]. Biomed Pharmacother, 2019, 114(2): 108-113. [2] Vander H, Steenbergen C. Cardioprotection and myocardial reperfusion[J]. Pitfalls to Clinical Application, 2013, 113(4): 464-477. [3] J. Xiong, F S Xue, Y J Yuan, et al. Cholinergic antiinflammatory pathway. A possible approach to protect against myocardial ischemia reperfusion injury[J], Chinese Medical Journa (Engl. ) , 2010, 123 (19) : 2720–2726. [4] H Fliss, D Gattinger. Apoptosis in ischemic and reperfused rat myocardium[J]. Circulation Research, 1996, 79(5): 949-956. [5] M Zhang, K Cheng, H Chen, et al. Galectin-3 knock down inhibits cardiac ischemia-reperfusion injury through interacting with bcl-2 and modulating cell apoptosis[J]. Arch Biochem Biophys, 2020, 694(3): 108-116. [6] Wang Z, Yao M, Jiang L, et al. DEXmedetomidine attenuates myocardial ischemia/reperfusion-induced ferroptosis via AMPK/GSK-3β/Nrf2 axis[J]. Biomed Pharmacother, 2022, 154(10): 772-786. [7] Zhou Y, Yang Y, Yi L, et al. Propofol and dexmedetomidine ameliorate endotoxemia-associated encephalopathy via inhibiting ferroptosis[J]. Drug Des Devel Ther, 2024, 18(10): 1349-1368. [8] Cao X, Zhao L, Zhou J, et al. Dexmedetomidine inhibits ferroptosis through the Akt/GSK3β/Nrf2 axis and alleviates adriamycin-induced cardiotoxicity[J]. Life Sci, 2025, 123(15): 609-616. [9] Feng M, Zheng C, Li X, et al. Reversal of lipopolysaccharide-induced cardiomyocyte apoptosis via α7nAChR by DEXmedetomidine[J]. Cellular & Molecular Biology Letters, 2023, 70(1): 1135-1139. [10] Ma X, Xu J, Gao N, et al. DEXmedetomidine attenuates myocardial ischemia-reperfusion injury via inhibiting ferroptosis by the cAMP/PKA/CREB pathway[J]. Molecular and Cellular Probes, 2023, 68(7): 1149-1167. [11] Wang Z, Yang Y, Xiong W, et al. DEXmedetomidine protects H9C2 against hypoxia/reoxygenation injury through miR-208b-3p/Med13/Wnt signaling pathway axis[J]. Biomedicine & Pharmacotherapy, 2020, 125(3): 123-131. [12] Deng X, Ye F, Zeng L, et al. DEXmedetomidine mitigates myocardial ischemia/reperfusion-induced mitochondrial apoptosis through targeting lncRNA HCP5[J]. American Journal of Chinese Medicine, 2022, 50(6): 371-382. [13] Cao X, Zhao L, Zhou J, et al. Dexmedetomidine inhibits ferroptosis through the Akt/GSK3β/Nrf2 axis and alleviates adriamycin-induced cardiotoxicity[J]. Life Sci, 2025, 371(7): 609-614. [14] Zhao Y S, Shi Y K, Li K F, et al. Dexmedetomidine regulates macrophage phenotype remodeling through AMPK/sirt1 to alleviate inflammatory mediators and lung injury[J]. Journal of Biochemical and Molecular Toxicology, 2025, 39(1): 701-708. [15] Yang K, Ma Y, Xie C, et al. Dexmedetomidine combined with propofol attenuates myocardial ischemia/reperfusion injury by activating the AMPK signaling pathway[J]. Biomedicine & Pharmacotherapy, 2023, 9(11): e22054. [16] Yang X, Wu J, Cheng H, et al. Dexmedetomidine ameliorates acute brain injury induced by myocardial ischemia-reperfusionvia upergulating the HIF-1 pathway[J]. Shock, 2023, 60(5): 678-687. [17] Avci O, Taskiran A S, Gundogdu, O. Dexmedetomidine, an α2 agonist, increases the morphine analgesic effect and decreases morphine tolerance development by suppressing oxidative stress and TNF/IL-1 signalling pathway in rats[J]. Rev Esp Anestesiol Reanim (Engl Ed), 2023, 70(6): 327-340. doi: 10.1016/j.redar.2022.04.003 [18] Yang K, Kong X, Xie C, et al. Combined administration of dexmedetomidine and propofol mitigates myocardial ischemia/reperfusion injury by modulating the Akt/mTOR/Nrf2 axis to suppress ferroptosis[J]. European Journal of Pharmacology, 2025, 997(1): 577-599. [19] Ning X, Tang J, Li X, et al. Dexmedetomidine ameliorates hepatic ischemia reperfusion injury via modulating SIRT3 mediated mitochondrial quality control[J]. Scientific Reports, 2025, 15(1): 5630-5638. doi: 10.1038/s41598-025-90069-1 [20] Wang L, Liu J, Wang Z, et al. DEXmedetomidine abates myocardial ischemia reperfusion injury through inhibition of pyroptosis via regulation of miR-665/MEF2D/Nrf2 axis[J]. Biomedicine & Pharmacotherapy, 2023, 165(3): 123-136. [21] Wang L, Tang S, Wang Z, et al. The administration of dexmedetomidine changes microRNA expression profiling of rat hearts[J]. Biomedicine & Pharmacotherapy, 2019, 120(10): 69-82. [22] He L, Wang Z, Zhou R, et al. DEXmedetomidine exerts cardioprotective effect through miR-146a-3p targeting IRAK1 and TRAF6 via inhibition of the NF-κB pathway[J]. Biomedicine & Pharmacotherapy, 2021, 133(5): 371-379. [23] Park J H, Oh J E, Kim N, et al. DEXmedetomidine alleviates CoCl2-induced hypoxic cellular damage in INS-1 cells by regulating autophagy[J]. Korean J Anesthesiol, 2023, 77(6): 609-617. [24] Chen S, Wu J, Li A, et al. Effect and mechanisms of DEXmedetomidine combined with macrophage migration inhibitory factor inhibition on the expression of inflammatory factors and AMPK in mice[J]. Clinical & Experimental Immunology, 2023, 212(12): 401-418. [25] Xu Y, Zhang X, Tang X, et al. Dexmedetomidine post-treatment exacerbates metabolic disturbances in septic cardiomyopathy via α2A-adrenoceptor[J]. Biomedicine & Pharmacotherapy, 2023, 170(11): 1993-2004. [26] Yin W, Wang C, Peng Y, et al. DEXmedetomidine alleviates H2O2-induced oxidative stress and cell necroptosis through activating of α2-adrenoceptor in H9C2 cells[J]. Molecular Biology Reports, 2020, 47(1): 371-380. [27] He Y, Yang Z Y, Li J L, et al. Dexmedetomidine reduces theinflammation and apoptosis of doxorubicin-induced myocardial cells[J]. Experimental and Molecular Pathology, 2020, 113(4): 104-111. [28] Baik J, Kim O, Jeon S, et al. Impact of nonselective and selective α-1 adrenergic blockers on the sedative efficacy of dexmedetomidine in urologic surgery: A prospective, observational study[J]. Medical Science Monitor, 2023, 29(9): 416-424. [29] Hou Z, Yang F, Chen K, et al. hUC-MSC-EV-miR-24 enhances the protective effect of DEXmedetomidine preconditioning against myocardial ischemia-reperfusion injury through the KEAP1/Nrf2/HO-1 signaling[J]. Drug Delivery and Translational Research, 2023, 14(3): 392-401. [30] Geng Q, Ainiwaer Y, Zhang J, et al. DEXmedetomidine alleviates myocardial injury induced by acute kidney injury in diabetes mellitus rats via regulating the inflammatory response[J]. Annals of Clinical and Laboratory Science, 2023, 54(4): 3829-3843. [31] Khan U, Hammer G B, Duncan-Azadi C, et al. A randomized, double-blind, dose-controlled study of the use of dexmedetomidine alone for procedural sedation of children and adolescents undergoing MRI scans[J]. Pediatric Anesthesia, 2024, 34(5): 405-414. , doi: 10.1111/pan.14857 [32] Zhang S, Tang J, Sun C et al. DEXmedetomidineattenu-ates hepatic ischemia-reperfusion injury-inducedapop-tosis via reducing oxidative stress and endoplasmicretic-ulum stress[J]. International Immunopharmacology, 2023, 117(8): 1757-1766. [33] Ding H, Liu D, He J, et al. The role of the Sirt1/Foxo3apathway in mitigating myocardial ischemia-reperfusion in-jury by dexmedetomidine[J]. Chem Biol Drug Des, 2025, 105(4): 701-710. [34] Yuan H, Guo J, Wang C, et al. Alleviation effects of DEXmedetomidine on myocardial ischemia/reperfusion injury through fatty acid metabolism pathway via Elovl6[J]. International Immunopharmacology, 2023, 138(2): 359-368. [35] Wang L, Tang S, Wang Z, et al. The administration of dexmedetomidine changes microRNA expression profiling of rat hearts[J]. Biomedicine & Pharmacotherapy, 2019, 120(11): 154-166. [36] Xion W, Zho R, Q Y, et al. Dexmedetomidine preconditioning mitigates myocardial ischemia/reperfusion injury via inhibition of mast cell degranulation[J]. Biomedicine & Pharmacotherapy, 2021, 141(6): 357-368. [37] Jiang L, Xiong W, Yang Y, et al. Insight into Cardioprotective effects and mechanisms of dexmedetomidine[J]. Journal of Cardiovascular Pharmacology and Therapeutics, 2024, 38(3): 428-441. [38] 黄颖, 董鸿捌, 陈云娥. 右美托咪定在重症患儿镇静镇痛的有效性和安全性分析[J]. 中国医药指南, 2024, 22(7): 5-9. [39] Shen J, Sun Y, Han D, et al. Effects of dexmedetomidine on perioperative cardiac adverse events in elderly patients with coronary heart disease[J]. Biomedicine & Pharmacotherapy, 2017, 42(10): 7692-7707. [40] 杨逸成, 陈贝儿, 叶凯雁, 等. 右美托咪定的心脏保护机制及其临床应用价值[J]. 中国医学科学院学报, 2022, 44(1): 130-135. [41] 黄佳佳, 郑吉建, 金立红. 右美托咪定滴鼻用于先天性心脏病患儿心脏彩色多普勒超声检查的镇静效果及成功率的影响因素分析[J]. 上海医学, 2023, 46(2): 87-91. [42] 段声吉. 右美托咪定预处理对异丙肾上腺素诱导的室性心律失常的影响及机制研究[D]. 南充: 川北医学院, 2023. -





 下载:
下载: