Gut-Brain Axis Mediated Mechanisms of SGLT-2Inhibitors in Enhancing Neural Repair after Ischemic Stroke
-
摘要:
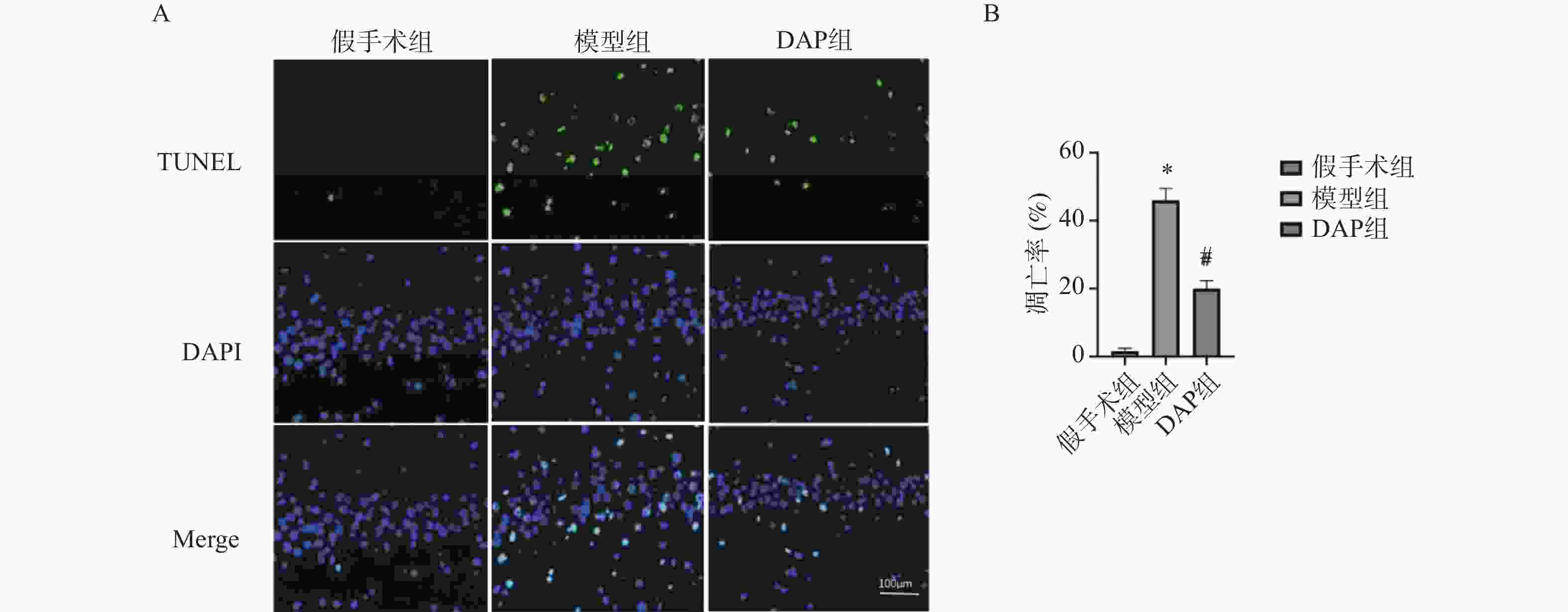
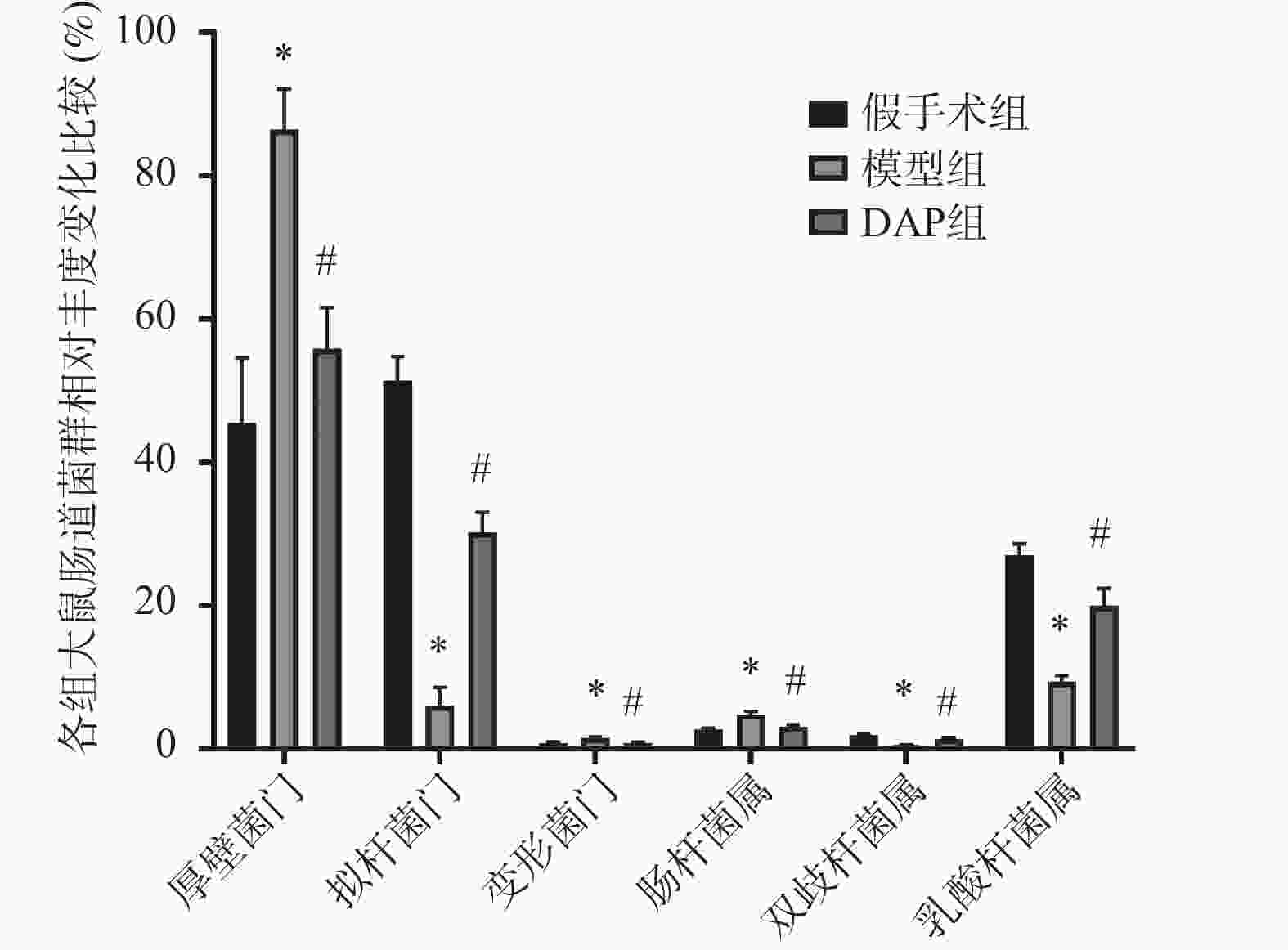
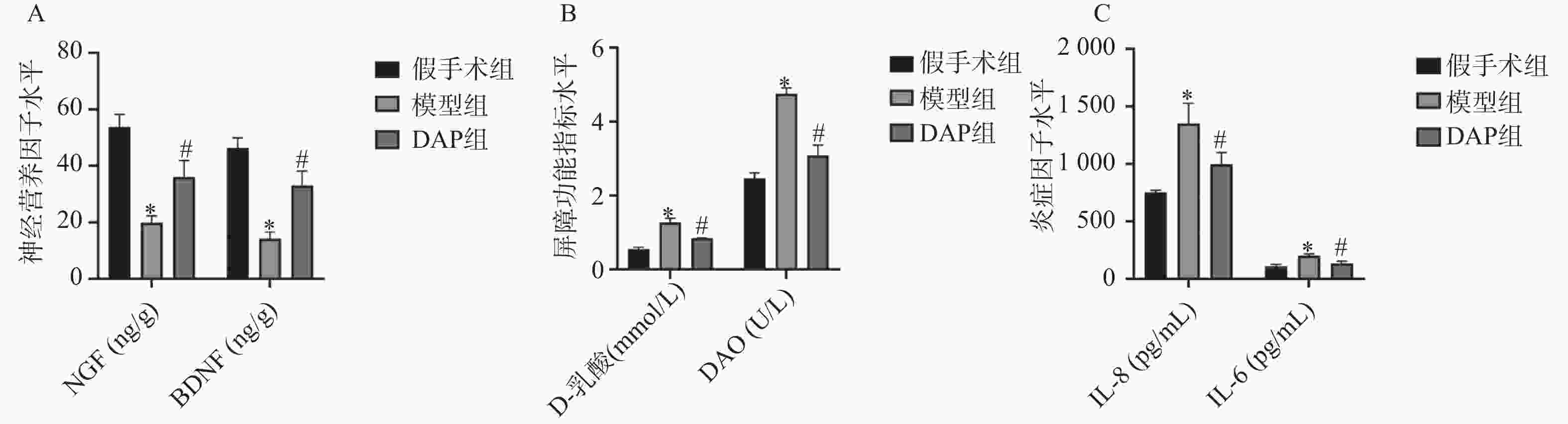
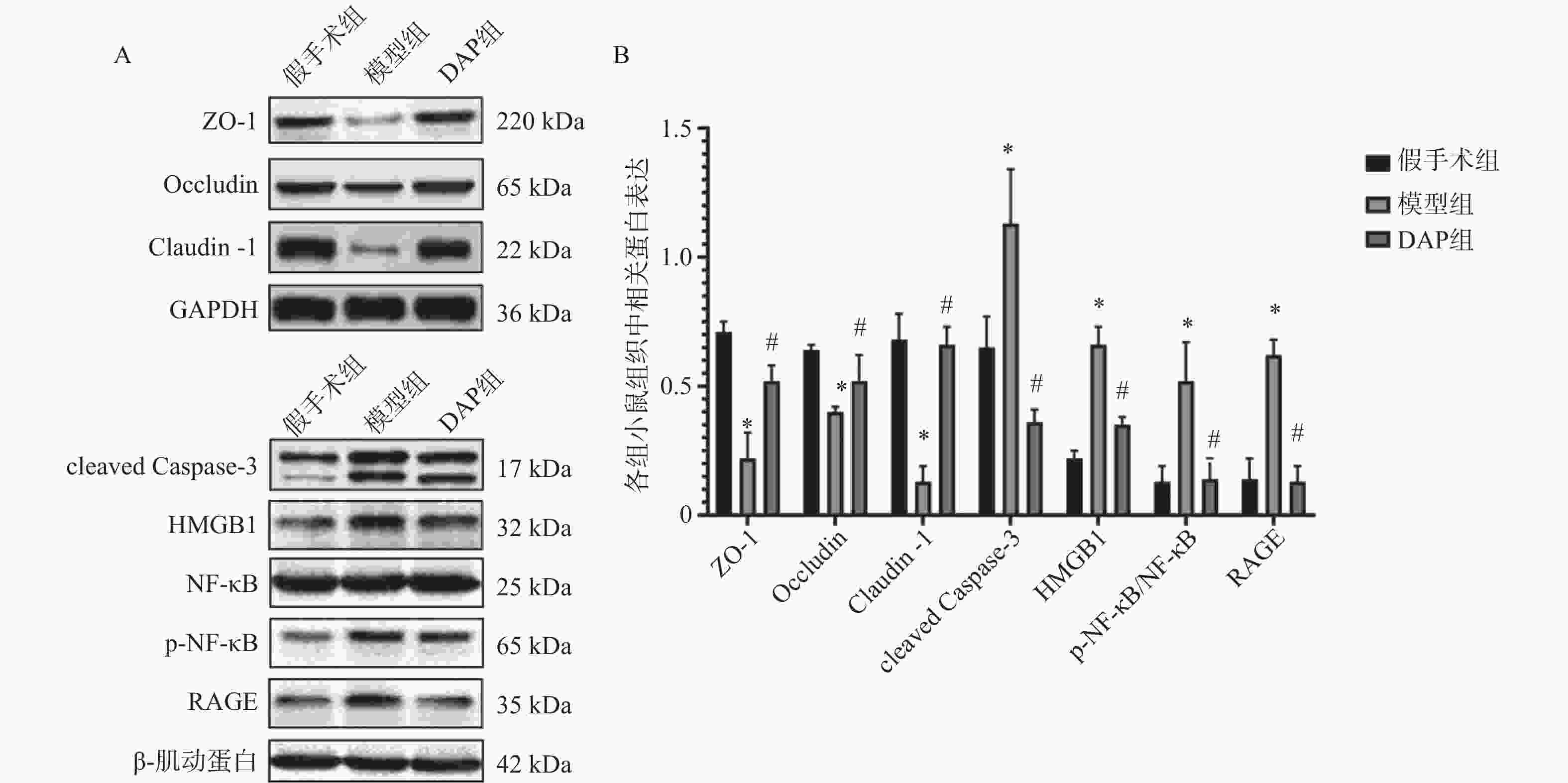
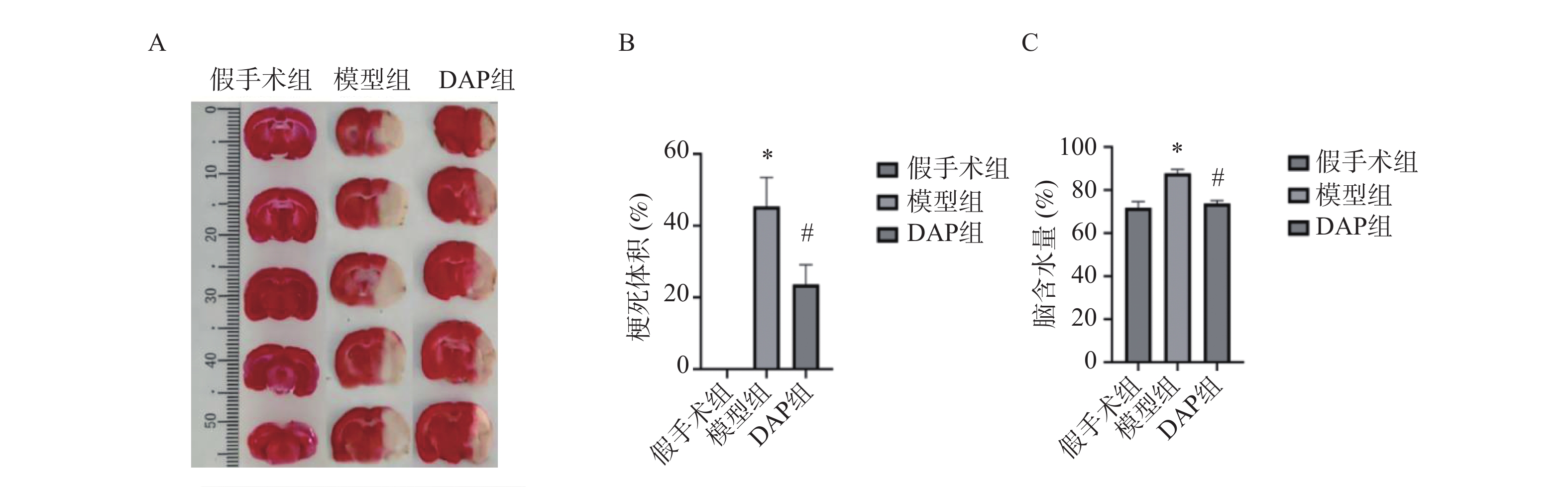
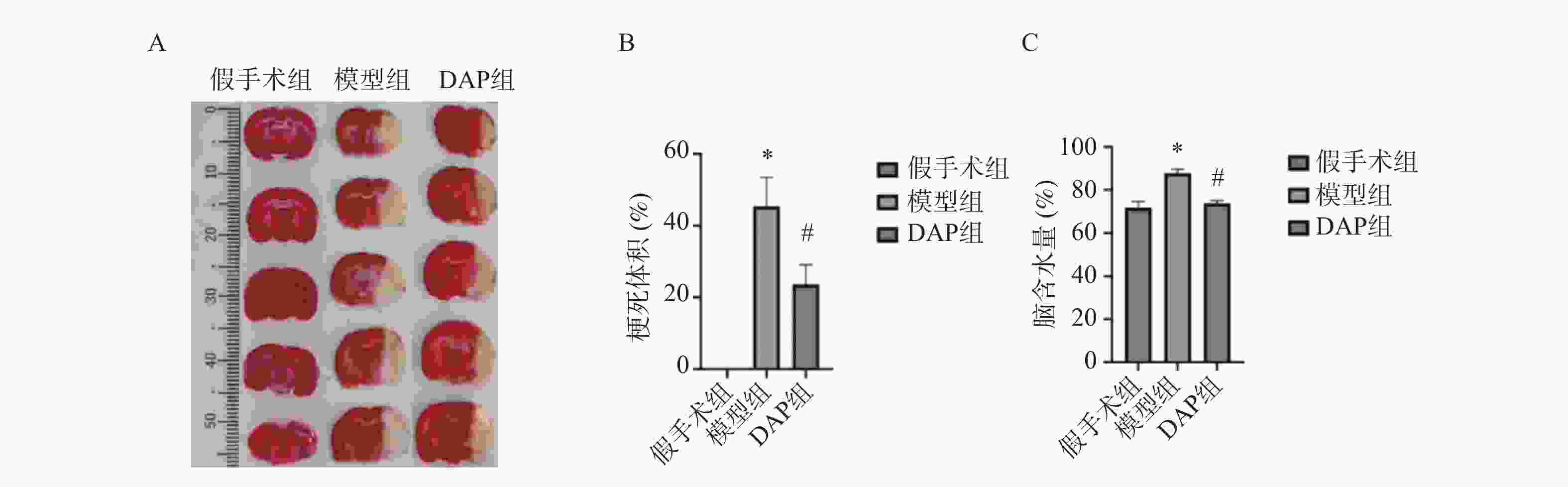
目的 探讨达格列净(dapagliflozin,DAP)对缺血性脑卒中(ischemic stroke,IS)大鼠大脑中动脉阻塞(middle cerebral artery occlusion,MCAO)模型的神经保护作用及机制。 方法 建立MCAO模型,将大鼠随机分为假手术组、模型组和DAP组(n = 10)。DAP组于再灌注2 h后灌胃[1 mg/(kg·d)],连续7 d。检测大鼠神经功能缺损评分(modified neurological severity score,mNSS)、脑梗死体积、脑含水量及海马神经元凋亡;16S核糖体RNA(16S ribosomal RNA,16S rRNA)测序分析菌群;酶联免疫吸附试验(enzyme linked immunosorbent assay,ELISA)测定血清神经生长因子(nerve growth factor,NGF)、脑源性神经营养因子(brain-derived neurotrophic factor,BDNF)、D-乳酸(D-lactic acid,D-LA)、二胺氧化酶(diamine oxidase,DAO)及多种炎症因子(inflammatory cytokines),包括肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、白细胞介素-1β(interleukin-1β,IL-1β)和白细胞介素-6(interleukin-6,IL-6)等;采用 Western blot 方法检测紧密连接蛋白1(zonula occludens-1,ZO-1)、闭锁蛋白(Occludin)、紧密连接蛋白 Claudin-1(claudin-1,CLDN-1)、切割型半胱天冬酶-3(cleaved caspase-3)、高级糖基化终产物相关核蛋白(high mobility group box 1,HMGB1)、高级糖基化终产物受体(receptor for advanced glycation end products,RAGE)、核因子κB(nuclear factor-κB,NF-κB)及磷酸化核因子κB(phosphorylated nuclear factor-κB,p-NF-κB)水平。 结果 与模型组相比,DAP组mNSS评分降低(P < 0.01),脑梗死体积、脑含水量及神经元凋亡减少(P < 0.01)。DAP组乳酸杆菌属(Lactobacillus)、双歧杆菌属(Bifidobacterium)丰度升高,肠杆菌属(Enterobacter)下降,并与mNSS相关(P < 0.001)。DAP提高血清NGF、BDNF,降低D-乳酸、DAO及炎症因子(P < 0.01),上调ZO-1、Occludin、Claudin-1,下调cleaved Caspase-3、HMGB1、RAGE、NF-κB及p-NF-κB(P < 0.01)。 结论 DAP可通过抑制凋亡与炎症、调节肠道菌群来改善MCAO大鼠神经功能和脑损伤。 Abstract:Objective To investigate the neuroprotective effects and underlying mechanisms of dapagliflozin (DAP) in a rat model of ischemic stroke (IS) induced by middle cerebral artery occlusion (MCAO). Methods The MCAO model was established and rats were randomly assigned to the sham, model, and DAP groups (n = 10). DAP (1 mg/kg·d) was administered by gavage 2 h after reperfusion for 7 consecutive days. Neurological function was assessed using the modified neurological severity score (mNSS). Infarct volume, brain water content, and hippocampal neuronal apoptosis were measured. Gut microbiota composition was analyzed by 16S rRNA sequencing. Serum levels of NGF, BDNF, D-lactate, DAO, and inflammatory cytokines were determined by ELISA. Protein expression of ZO-1, Occludin, Claudin-1, cleaved Caspase-3, HMGB1, RAGE, NF-κB, and p-NF-κB was detected by Western blot. Results Compared with the model group, DAP significantly reduced mNSS scores, (P < 0.01) infarct volume, brain water content, and neuronal apoptosis ( P < 0.01). 16S rRNA sequencing revealed increased abundances of Lactobacillus and Bifidobacterium, and decreased Enterobacter in the DAP group, which were significantly correlated with mNSS scores (P < 0.001). DAP increased serum NGF and BDNF levels, while reducing D-lactate, DAO, and inflammatory cytokines (all P < 0.01). Western blot showed that DAP upregulated ZO-1, Occludin, and Claudin-1, while downregulating cleaved Caspase-3, HMGB1, RAGE, NF-κB, and p-NF-κB (all P < 0.01). Conclusion DAP can improve neurological function and brain injury in MCAO rats by inhibiting apoptosis and inflammation and regulating intestinal flora. -
Key words:
- Ischemic Stroke /
- Dapagliflozin /
- Gut–brain Axis /
- Gut Microbiota /
- Neuroprotection
-
表 1 各组大鼠mNSS评分比较($ \bar x \pm s $,n = 10)
Table 1. Comparison of mNSS scores in groups of rats ($ \bar x \pm s $,n = 10)
组别 假手术组 模型组 DAP组 mNSS评分(分) 0.00 ± 0.00 13.06 ± 0.73* 6.50 ± 0.54 # 与假手术组比较,*P < 0.01;与模型组比较,#P < 0.01。 表 2 各组大鼠菌群相对丰度与mNSS评分的相关性关系
Table 2. Correlation analysis between relative abundance of bacterial flora and mNSS score in rats
菌群 神经功能评分 r P 双歧杆菌属 −0.896 <0.001 乳酸杆菌属 −0.926 <0.001 肠杆菌属 0.922 <0.001 -
[1] Ding Q, Liu S, Yao Y, et al. Global, regional, and national burden of ischemic stroke, 1990–2019[J]. Neurology, 2022, 98(3): e279-e290. [2] Zhao Y, Zhang X, Chen X, et al. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment[J]. Int J Mol Med, 2022, 49(2): 1-9. [3] Mira R G, Lira M, Cerpa W. Traumatic brain injury: mechanisms of glial response[J]. Front Physiol, 2021, 12: 740939. doi: 10.3389/fphys.2021.740939 [4] Pan Y, Shi G. Silver jubilee of stroke thrombolysis with alteplase: evolution of the therapeutic window[J]. Front Neurol, 2021, 12: 593887. doi: 10.3389/fneur.2021.593887 [5] Fischesser D M, Bo B, Benton R P, et al. Controlling reperfusion injury with controlled reperfusion: Historical perspectives and new paradigms[J]. J Cardiovasc Pharmacol Ther, 2021, 26(6): 504-523. doi: 10.1177/10742484211046674 [6] Zhou S Y, Guo Z N, Yang Y, et al. Gut-brain axis: Mechanisms and potential therapeutic strategies for ischemic stroke through immune functions[J]. Front Neurosci, 2023, 17: 1081347. doi: 10.3389/fnins.2023.1081347 [7] Huang Q, Xia J. Influence of the gut microbiome on inflammatory and immune response after stroke[J]. Neurol Sci, 2021, 42(12): 4937-4951. doi: 10.1007/s10072-021-05603-6 [8] Liu Y, Zhao P, Cai Z, et al. Buqi-Huoxue-Tongnao decoction drives gut microbiota-derived indole lactic acid to attenuate ischemic stroke via the gut-brain axis[J]. Chin Med, 2024, 19(1): 126. doi: 10.1186/s13020-024-00991-1 [9] Zou X, Wang L, Xiao L, et al. Gut microbes in cerebrovascular diseases: gut flora imbalance, potential impact mechanisms and promising treatment strategies[J]. Front Immunol, 2022, 13: 975921. doi: 10.3389/fimmu.2022.975921 [10] Kamp Y J M, de Ligt M, Dautzenberg B, et al. Effects of the SGLT2 inhibitor dapagliflozin on energy metabolism in patients with type 2 diabetes: a randomized, double-blind crossover trial[J]. Diabetes Care, 2021, 44(6): 1334-1343. doi: 10.2337/dc20-2887 [11] Brown E, Wilding J P H, Alam U, et al. The expanding role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection[J]. Ann Med, 2021, 53(1): 2072-2089. doi: 10.1080/07853890.2020.1841281 [12] Giglio R V, Patti A M, Rizvi A A, et al. Advances in the pharmacological management of diabetic nephropathy: a 2022 international update[J]. Biomedicines, 2023, 11(2): 291. doi: 10.3390/biomedicines11020291 [13] Chidambaram S B, Rathipriya A G, Mahalakshmi A M, et al. The influence of gut dysbiosis in the pathogenesis and management of ischemic stroke[J]. Cells, 2022, 11(7): 1239. doi: 10.3390/cells11071239 [14] Biose I J, Chastain W H, Wang H, et al. Optimizing intraluminal monofilament model of ischemic stroke in middle-aged sprague-dawley rats[J]. BMC Neurosci, 2022, 23(1): 75. doi: 10.1186/s12868-022-00764-2 [15] Nuszkiewicz J, Kukulska-Pawluczuk B, Piec K, et al. Intersecting pathways: the role of metabolic dysregulation, gastrointestinal microbiome, and inflammation in acute ischemic stroke pathogenesis and outcomes[J]. J Clin Med, 2024, 13(14): 4258. doi: 10.3390/jcm13144258 [16] Simats A, Liesz A. Systemic inflammation after stroke: implications for post‐stroke comorbidities[J]. EMBO Mol Med, 2022, 14(9): e16269. doi: 10.15252/emmm.202216269 [17] Saeed M M. Repurposing dapagliflozin for Alzheimer's disease: a mechanistic exploration[J]. Future J Pharm Sci, 2024, 10(1): 177. doi: 10.1186/s43094-024-00751-w [18] Pham L T T, Mangmool S, Parichatikanond W. Sodium-glucose cotransporter 2 (SGLT2) inhibitors: Guardians against mitochondrial dysfunction and endoplasmic reticulum stress in heart diseases[J]. ACS Pharmacol Transl Sci, 2024, 7(11): 3279-3298. doi: 10.1021/acsptsci.4c00240 [19] Huang X, Hussain B, Chang J. Peripheral inflammation and blood–brain barrier disruption: effects and mechanisms[J]. CNS Neurosci Ther, 2021, 27(1): 36-47. doi: 10.1111/cns.13569 [20] Sakaue T, Fujishima Y, Fukushima Y, et al. Adiponectin accumulation in the retinal vascular endothelium and its possible role in preventing early diabetic microvascular damage[J]. Sci Rep, 2022, 12(1): 4159. doi: 10.1038/s41598-022-08041-2 [21] Zhang X, Li H, Zhao Y, et al. Neuronal injury after ischemic stroke: Mechanisms of crosstalk involving necroptosis[J]. J Mol Neurosci, 2025, 75(1): 15. doi: 10.1007/s12031-025-02313-y [22] Li N, Zhu Q X, Li G Z, et al. Empagliflozin ameliorates diabetic cardiomyopathy probably via activating AMPK/PGC-1α and inhibiting the RhoA/ROCK pathway[J]. World J Diabetes, 2023, 14(12): 1862. doi: 10.4239/wjd.v14.i12.1862 [23] Zhang J X, Yuan W C, Li C G, et al. A review on the mechanisms underlying the antitumor effects of natural products by targeting the endoplasmic reticulum stress apoptosis pathway[J]. Front Pharmacol, 2023, 14: 1293130. doi: 10.3389/fphar.2023.1293130 [24] Xie W, Yan X, Yang X, et al. The regulation of neuroinflammatory response after stroke by intestinal flora microorganisms[J]. Front Cell Infect Microbiol, 2025, 15: 1594834. doi: 10.3389/fcimb.2025.1594834 [25] Gong X, Liu Y, Liu X, et al. Disturbance of gut bacteria and metabolites are associated with disease severity and predict outcome of NMDAR encephalitis: A prospective case–control study[J]. Front Immunol, 2022, 12: 791780. doi: 10.3389/fimmu.2021.791780 [26] Lu Q, Guo Y, Yang G, et al. Structure and anti-inflammation potential of lipoteichoic acids isolated from Lactobacillus strains[J]. Foods, 2022, 11(11): 1610. doi: 10.3390/foods11111610 [27] Jing Y, Yang D, Bai F, et al. Spinal cord injury-induced gut dysbiosis influences neurological recovery partly through short-chain fatty acids[J]. NPJ Biofilms Microbiomes, 2023, 9(1): 99. doi: 10.1038/s41522-023-00466-5 [28] Yang L, Li X, Zhu X, et al. Involvement of role of HMGB1-NLRP3 pathway in systemic disorders[J]. Front Cell Dev Biol, 2025, 13: 1600596. doi: 10.3389/fcell.2025.1600596 -





 下载:
下载: