FGF2 Regulates Hypoxia-Induced Proliferation and Collagen Metabolism of Scleral Fibroblasts Through the PERK/EIF2α/ATF4 Signaling Pathway
-
摘要:
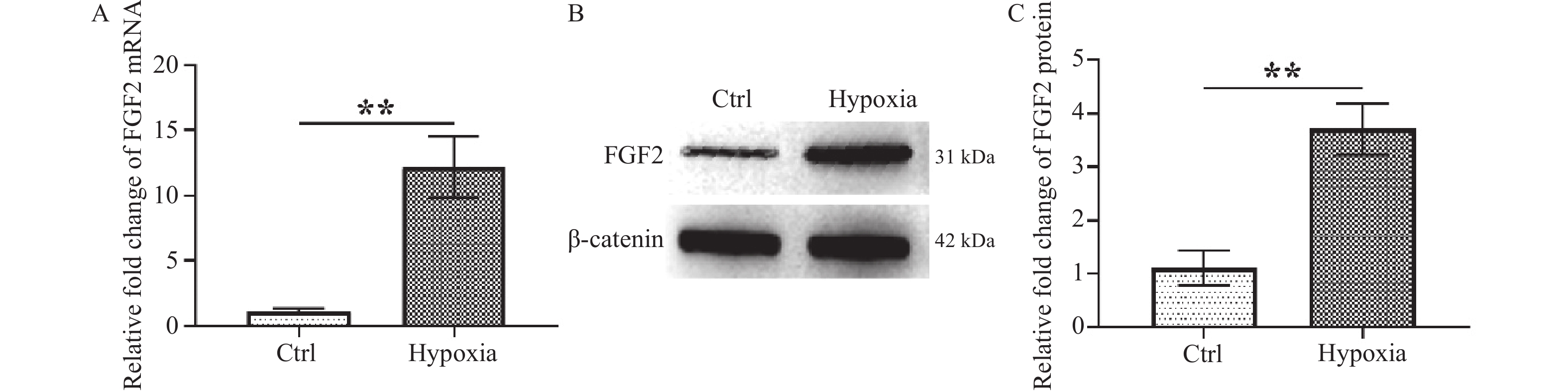
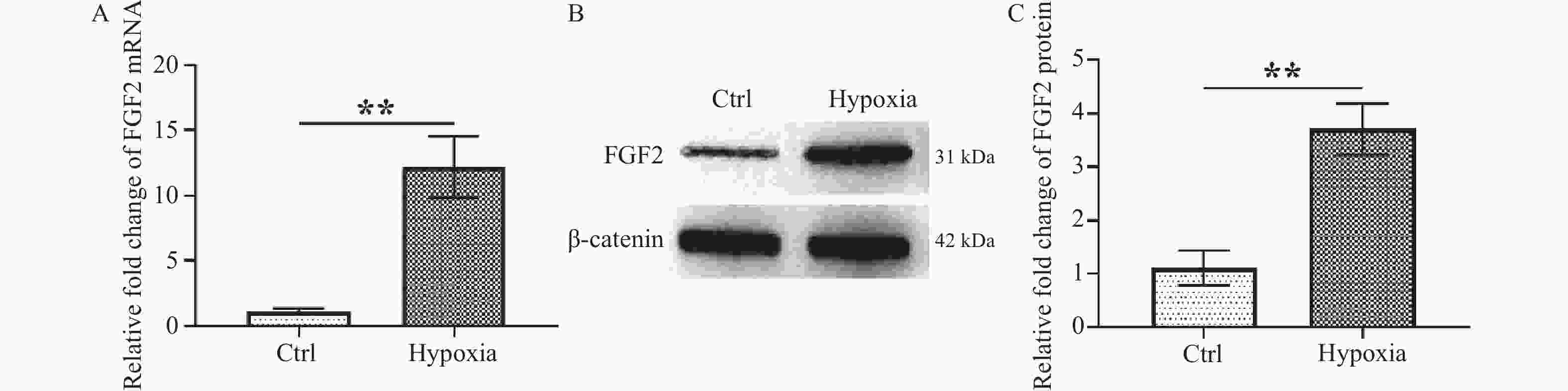
目的 研究FGF2对缺氧诱导的巩膜成纤维细胞(scleral fibroblasts,SF)增殖和胶原的影响并探讨其可能调节的下游信号通路。 方法 用5% O2刺激SF 24 h诱导近视SF模型,RT-qPCR检测FGF2 mRNA表达,Western blot检测FGF2蛋白表达。细胞计数试剂盒8(Cell Count Kit-8,CCK-8)、流式细胞术和Western blot分别检测细胞增殖活力、细胞凋亡和胶原代谢相关蛋白collagen Ⅰ、MMP2以及通路蛋白PERK、p-PERK、EIF2α、EIF2α、ATF4表达。 结果 缺氧刺激可促进FGF2 mRNA和蛋白表达升高(P < 0.01)并激活PERK/EIF2α/ATF4通路(P < 0.001),抑制SF细胞增殖(P < 0.001)和collagen Ⅰ表达(P < 0.001),诱导MMP2表达(P < 0.001)及细胞凋亡(P < 0.001)。敲降FGF2或经PERK抑制剂GSK2606414处理可逆转缺氧对SF细胞的作用,促进细胞增殖活力(P < 0.001)和collagen Ⅰ表达(P < 0.01),减少细胞凋亡(P < 0.01)。 结论 FGF2通过调节PERK/EIF2α/ATF4通路的激活,影响缺氧诱导的SF增殖及胶原代谢。 Abstract:Objectives To investigate the effects of FGF2 on the proliferation and collagen production of hypoxia-induced scleral fibroblasts (SF) and explore the downstream signaling pathways it regulates. Methods 5% O2 was used to stimulate the SF to induce myopia SF model for 24 hours. RT-qPCR was used to detect FGF2 mRNA expression, and Western blot analysis was used to check FGF2 protein expression. The Cell Counting Kit-8 (CCK-8), flow cytometry, and Western blot were used to assess cell proliferation vitality, cell apoptosis, and the expression of collagen metabolism-related proteins collagen I, MMP2, and pathway proteins PERK, p-PERK, EIF2α, EIF2α, and ATF4. Results Hypoxia increased FGF2 mRNA and protein expression (P < 0.01) , activated the PERK/EIF2α/ATF4 pathway (P < 0.001), inhibited SF cell proliferation (P < 0.001) and collagen I expression (P < 0.001), while induced MMP2 expression (P < 0.001) and apoptosis (P < 0.001). Knocking down FGF2 or treating with PERK inhibitor GSK2606414 reversed the effect of hypoxia on SF cells, increased cell proliferation (P < 0.001) and collagen Ⅰ expression (P < 0.01), and suppressed cell apoptosis (P < 0.01). Mechanism study revealed that FGF2 knockdown dampened the activation of PERK/EIF2α/ATF4 pathway. Conclusion FGF2 affects hypoxia-induced SF proliferation and collagen metabolism by regulating the activation of PERK/EIF2α/ATF4 signaling pathway. -
Key words:
- Scleral fibroblasts /
- Hypoxia /
- FGF2 /
- Proliferation /
- Collagen metabolism
-
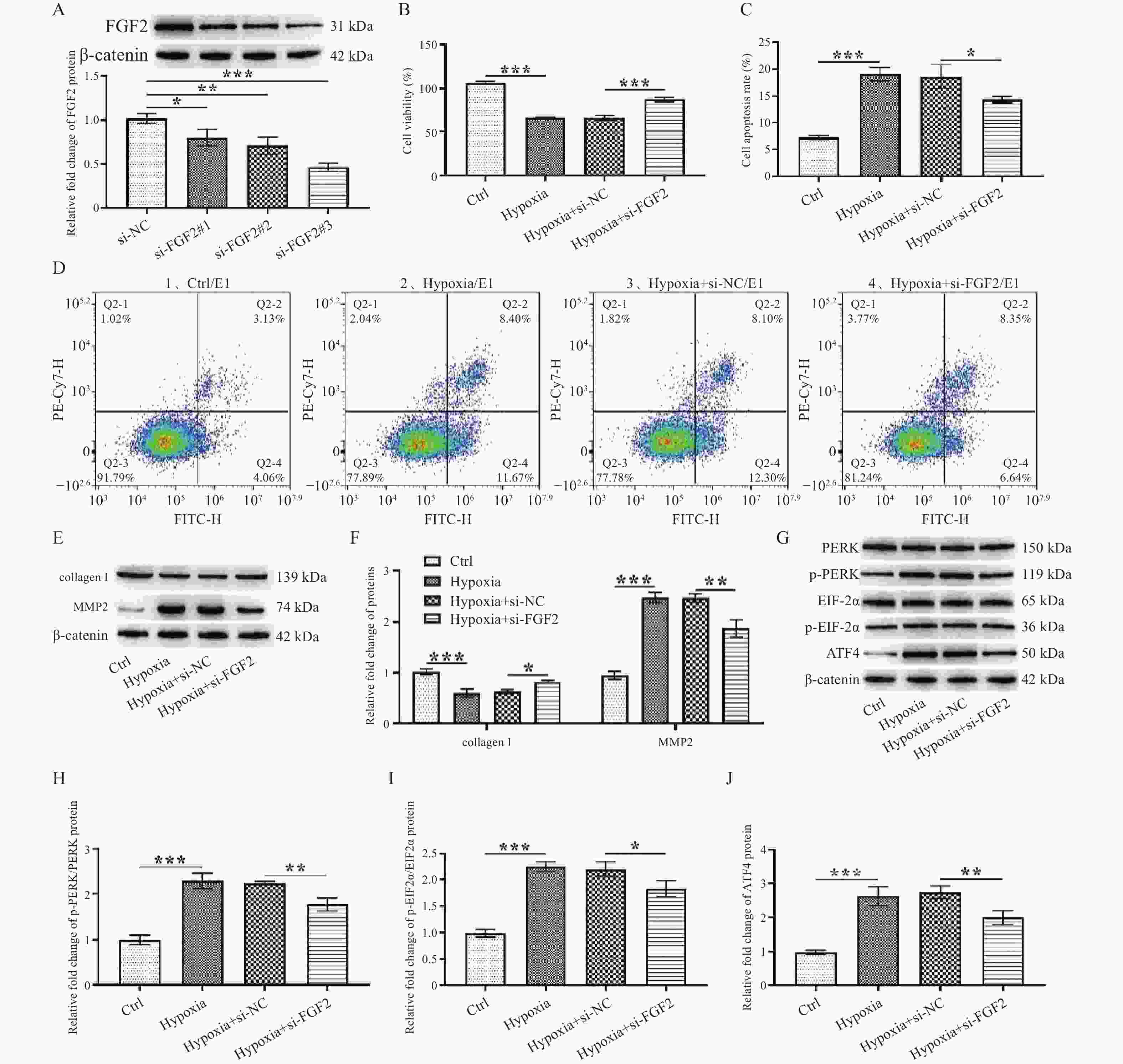
图 2 FGF2调控缺氧诱导的SF增殖和胶原代谢
A:转染si-FGF2的SF中FGF2蛋白相对表达水平;B:细胞增殖活力;C~D:细胞凋亡率;E:collagen Ⅰ和MMP2蛋白表达电泳图;F:collagen Ⅰ和MMP2蛋白表达统计学分析;G:PERK、p-PERK、EIF2α、p-EIF2α、ATF4蛋白表达电泳图;H:p-PERK/PERK蛋白表达统计学分析;I:p-EIF2α/EIF2α蛋白表达统计学分析;J:ATF4蛋白表达统计学分析。*P < 0.05,**P < 0.01,***P < 0.001。Ctrl:正常对照组,Hypoxia:缺氧组。
Figure 2. FGF2 regulates proliferation and collagen metabolism in hypoxia-induced SF
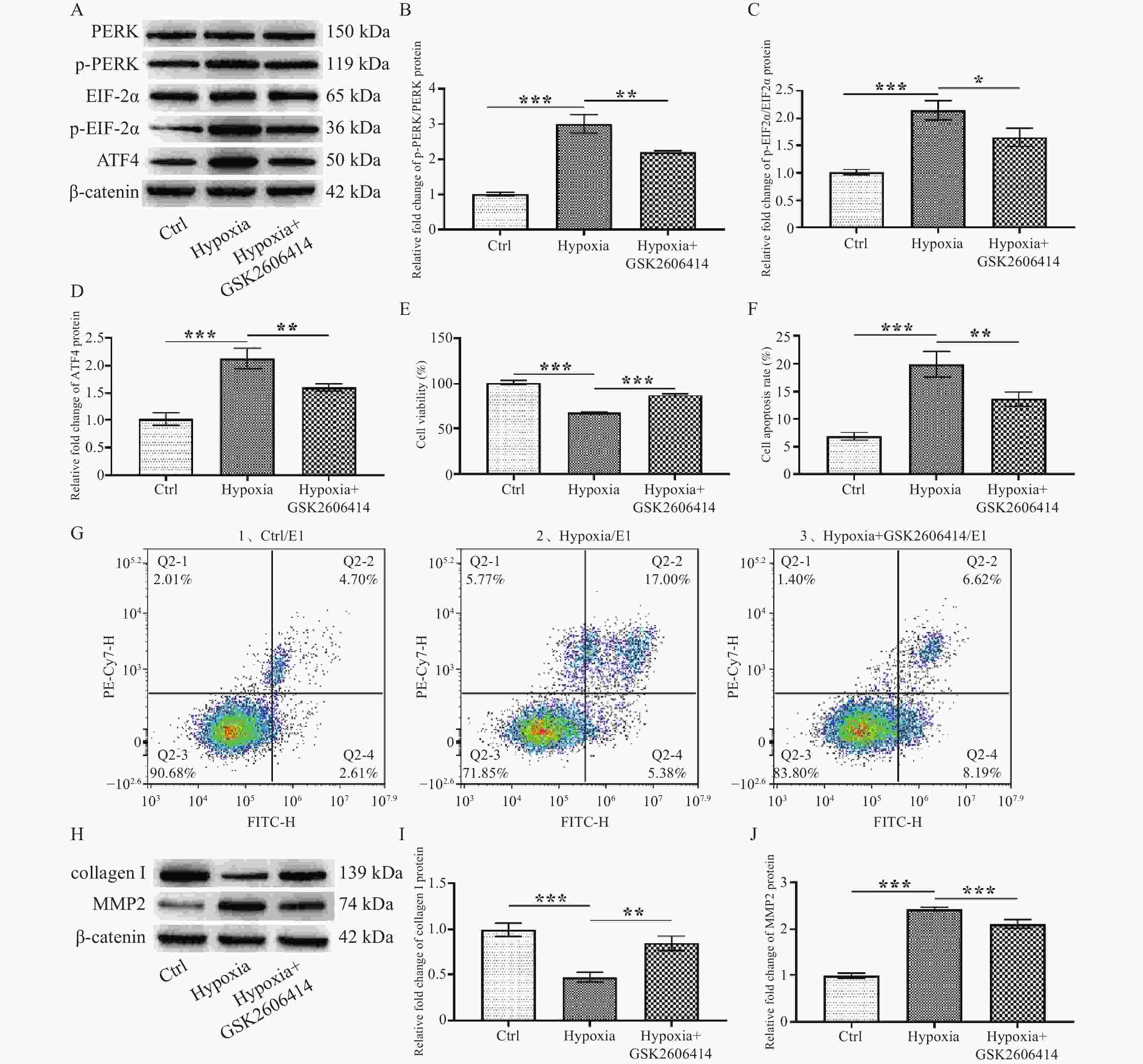
图 3 PERK/EIF2α/ATF4通路调控缺氧诱导的SF增殖及胶原代谢
A:PERK、p-PERK、EIF2α、p-EIF2α、ATF4蛋白的蛋白表达电泳图;B:p-PERK/PERK蛋白表达统计学分析;C:p-EIF2α/EIF2α蛋白表达统计学分析;D:ATF4蛋白表达统计学分析;E:细胞增殖活力;F:细胞凋亡率统计;G:细胞凋亡图;H:collagen Ⅰ和MMP2蛋白表达电泳图:I:collagen Ⅰ蛋白表达统计学分析;J:MMP2蛋白表达统计学分析。*P < 0.05,**P < 0.01,***P < 0.001。Ctrl:正常对照组,Hypoxia:缺氧组。
Figure 3. PERK/EIF2α/ATF4 pathway regulates proliferation and collagen metabolism in hypoxia-induced SF
表 1 引物序列
Table 1. Primer sequences
目的基因 引物序列
(F: Forward,R: Reverse,5’-3’)FGF2 F: GTCAAACTACAACTCCAAGCAG R: GAAACACTCTTCTGTAACACACTT GAPDH F: GTGGAGTCATACTGGAACATGTAG R: AATGGTGAAGGTCGGTGTG -
[1] Karthikeyan S K,Ashwini D L,Priyanka M,et al. Physical activity,time spent outdoors,and near work in relation to myopia prevalence,incidence,and progression: An overview of systematic reviews and meta-analyses[J]. Indian J Ophthalmol,2022,70(3):728-739. doi: 10.4103/ijo.IJO_1564_21 [2] Karl A,Makarov F N,Koch C,et al. The ultrastructure of rabbit sclera after scleral crosslinking with riboflavin and blue light of different intensities[J]. Graefes Arch Clin Exp Ophthalmol,2016,254(8):1567-1577. doi: 10.1007/s00417-016-3393-z [3] Shi W Q,Li T,Liang R,et al. Targeting scleral remodeling and myopia development in form deprivation myopia through inhibition of EFEMP1 expression[J]. Biochim Biophys Acta Mol Basis Dis,2024,1870(3):166981. doi: 10.1016/j.bbadis.2023.166981 [4] Lin X,Lei Y,Pan M,et al. Augmentation of scleral glycolysis promotes myopia through histone lactylation[J]. Cell Metab,2024,36(3): 511-525. e7. [5] Xue M,Li B,Lu Y,et al. FOXM1 participates in scleral remodeling in myopia by upregulating APOA1 expression through METTL3/YTHDF2[J]. Invest Ophthalmol Vis Sci,2024,65(1): 19. [6] Wang X,Hui Q,Jin Z,et al. Roles of growth factors in eye development and ophthalmic diseases[J]. Zhejiang Da Xue Xue Bao Yi Xue Ban,2022,51(5):613-625. [7] Qin Y,Liu T,Zhang Z,et al. Scleral remodeling in early adulthood: the role of FGF-2[J]. Sci Rep,2023,13(1):20779. doi: 10.1038/s41598-023-48264-5 [8] An J,Hsi E,Zhou X,et al. The FGF2 gene in a myopia animal model and human subjects[J]. Mol Vis,2012,18:471-478. [9] Kolodeeva O E, Kolodeeva O E, Averinskaya D A, et al. Induction of the PERK-eIF2α-ATF4 pathway in M1 macrophages under endoplasmic reticulum stress[J]. Dokl Biochem Biophys, 2024, 517(1): 264-268.Kolodeeva O E,Kolodeeva O E,Averinskaya D A,et al. Induction of the PERK-eIF2α-ATF4 pathway in M1 macrophages under endoplasmic reticulum stress[J]. Dokl Biochem Biophys,2024,517(1): 264-268. [10] Wu H,Chen W,Zhao F,et al. Scleral hypoxia is a target for myopia control[J]. Proc Natl Acad Sci U S A,2018,115(30):7091-7100. [11] Ikeda S I,Kurihara T,Jiang X,et al. Scleral PERK and ATF6 as targets of myopic axial elongation of mouse eyes[J]. Nat Commun,2022,13(1):5859. doi: 10.1038/s41467-022-33605-1 [12] Holden B A,Fricke T R,Wilson D A,et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050[J]. Ophthalmology,2016,123(5):1036-1042. doi: 10.1016/j.ophtha.2016.01.006 [13] Cooper J,Tkatchenko A V. A Review of current concepts of the etiology and treatment of myopia[J]. Eye Contact Lens,2018,44(4):231-247. doi: 10.1097/ICL.0000000000000499 [14] Wang X,Fan W,Li N,et al. YY1 lactylation in microglia promotes angiogenesis through transcription activation-mediated upregulation of FGF2[J]. Genome Biol,2023,24(1):87. doi: 10.1186/s13059-023-02931-y [15] Lee J,Jung E,Heur M. Injury induces endothelial to mesenchymal transition in the mouse corneal endothelium in vivo via FGF2[J]. Mol Vis,2019,25:22-34. [16] Lee J G,Heur M. Interleukin-1β enhances cell migration through AP-1 and NF-κB pathway-dependent FGF2 expression in human corneal endothelial cells[J]. Biol Cell,2013,105(4):175-189. doi: 10.1111/boc.201200077 [17] Flokis M,Lovicu F J. FGF-2 Differentially Regulates Lens Epithelial Cell Behaviour during TGF-β-Induced EMT[J]. Cells,2023,12(6):110. [18] Shao Z,Wu J,Du G,et al. Young bone marrow Sca-1 cells protect aged retina from ischaemia-reperfusion injury through activation of FGF2[J]. J Cell Mol Med,2018,22(12):6176-6189. doi: 10.1111/jcmm.13905 [19] Walter P,Ron D. The unfolded protein response: from stress pathway to homeostatic regulation[J]. Science,2011,334(6059):1081-1086. doi: 10.1126/science.1209038 [20] Wan H,Wang Q,Chen X,et al. WDR45 contributes to neurodegeneration through regulation of ER homeostasis and neuronal death[J]. Autophagy,2020,16(3):531-547. doi: 10.1080/15548627.2019.1630224 [21] Raines L N,Zhao H,Wang Y,et al. PERK is a critical metabolic hub for immunosuppressive function in macrophages[J]. Nat Immunol,2022,23(3):431-445. doi: 10.1038/s41590-022-01145-x [22] Shi S,Ding C,Zhu S,et al. PERK inhibition suppresses neovascularization and protects neurons during ischemia-induced retinopathy[J]. Invest Ophthalmol Vis Sci,2023,64(11):17. doi: 10.1167/iovs.64.11.17 -





 下载:
下载: