Intervention of AKR1C3 on Malignant Biological Behavior of Breast Cancer Cells through PD1/PD-L1 Signaling Pathway
-
摘要:
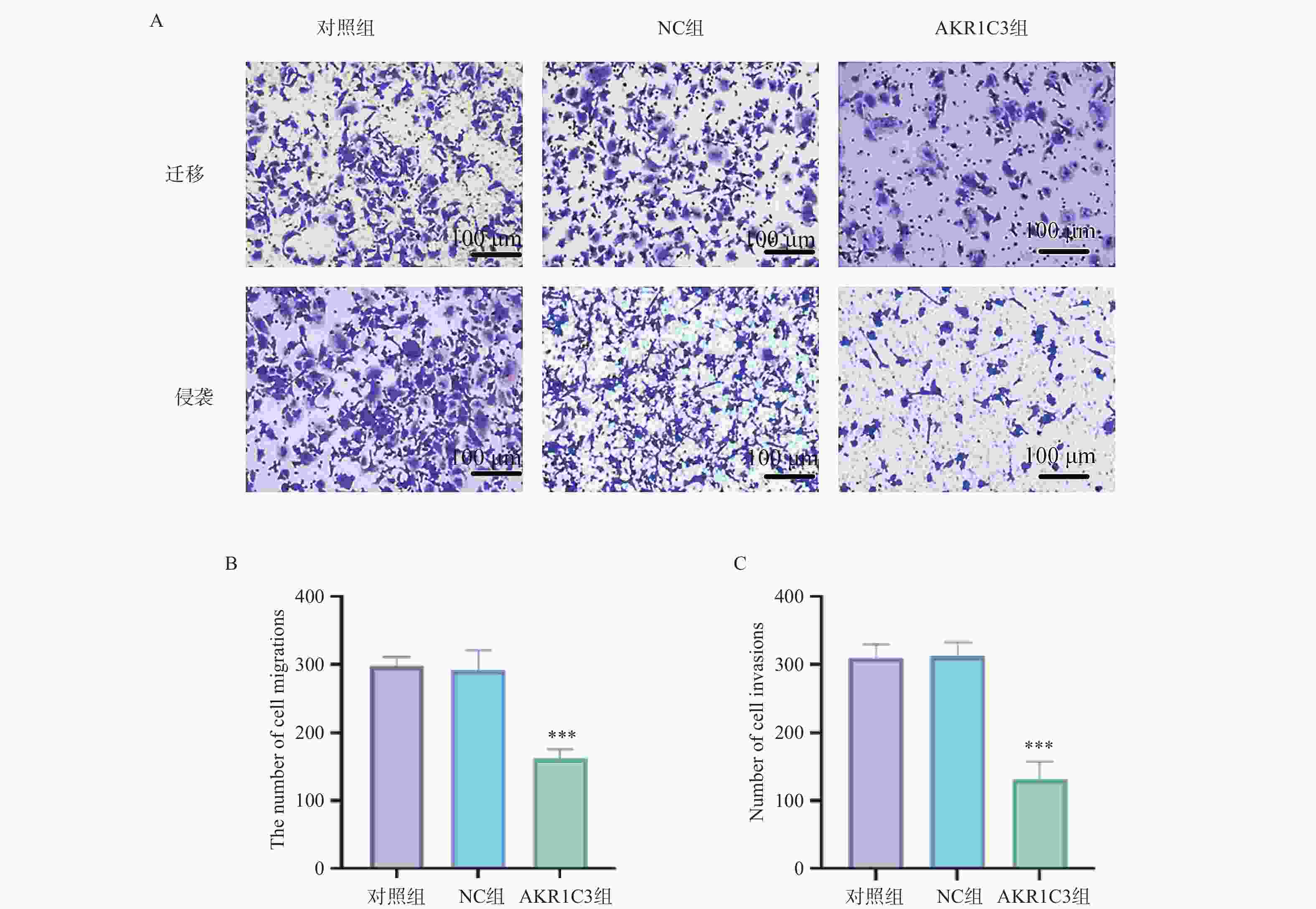
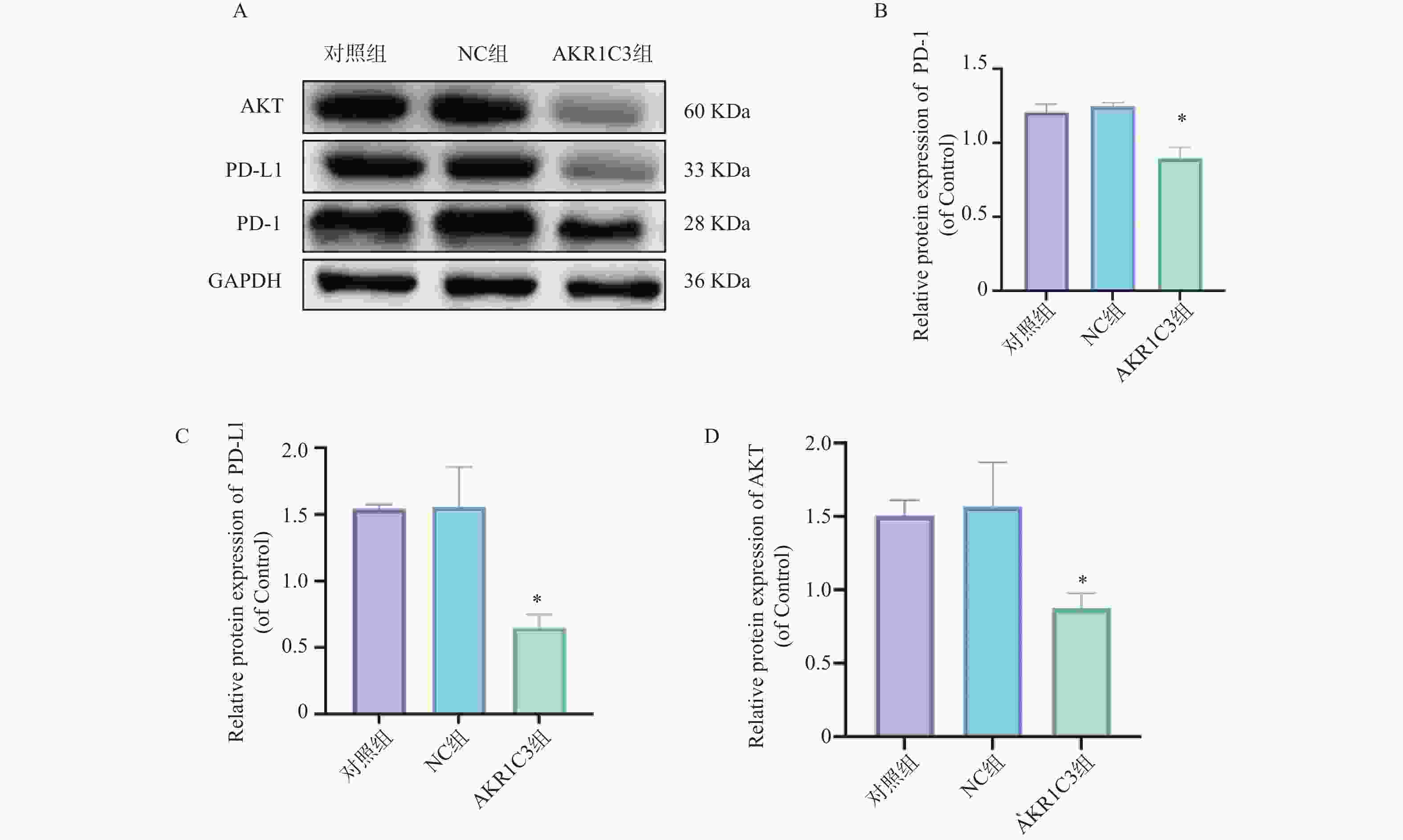
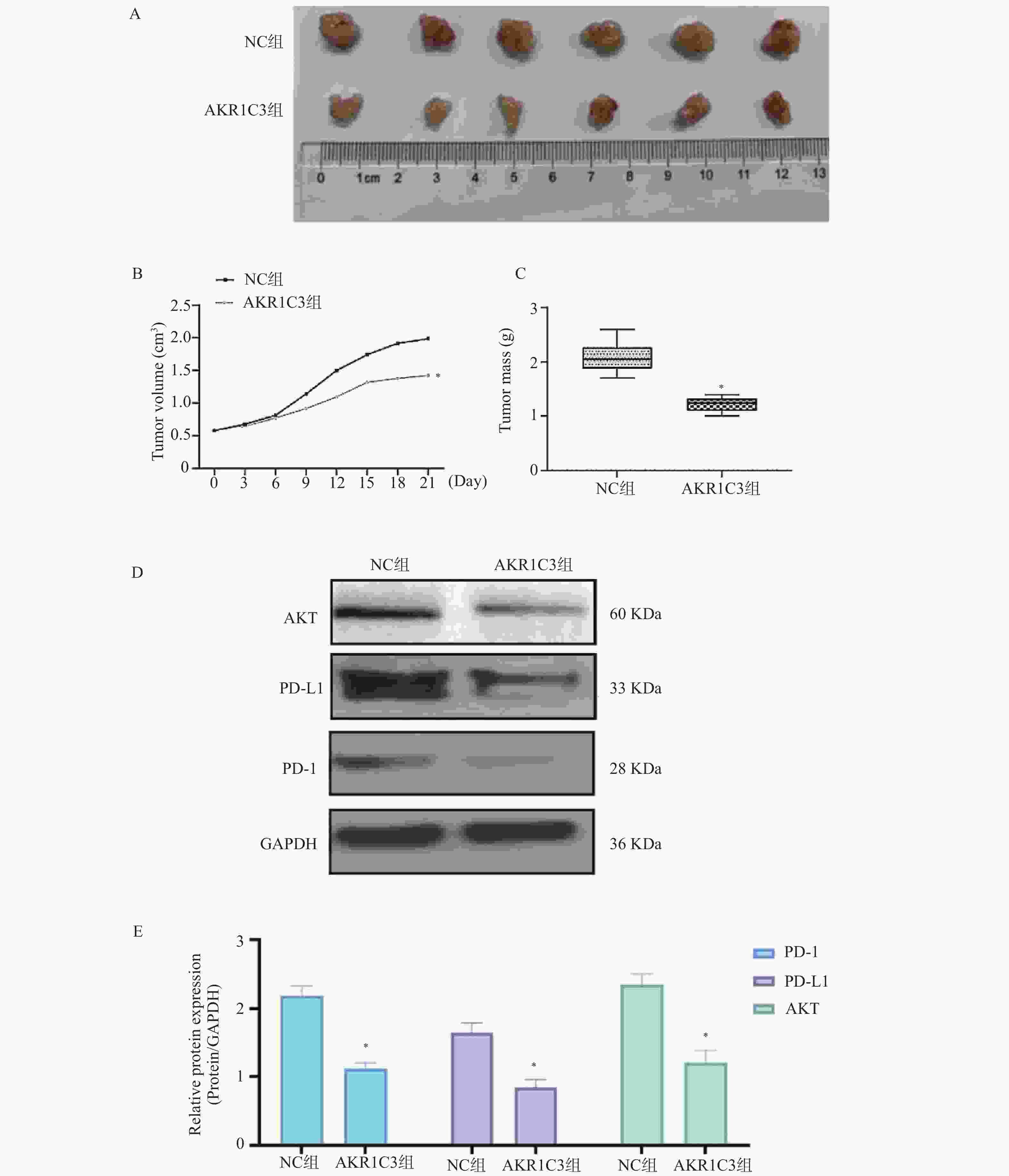
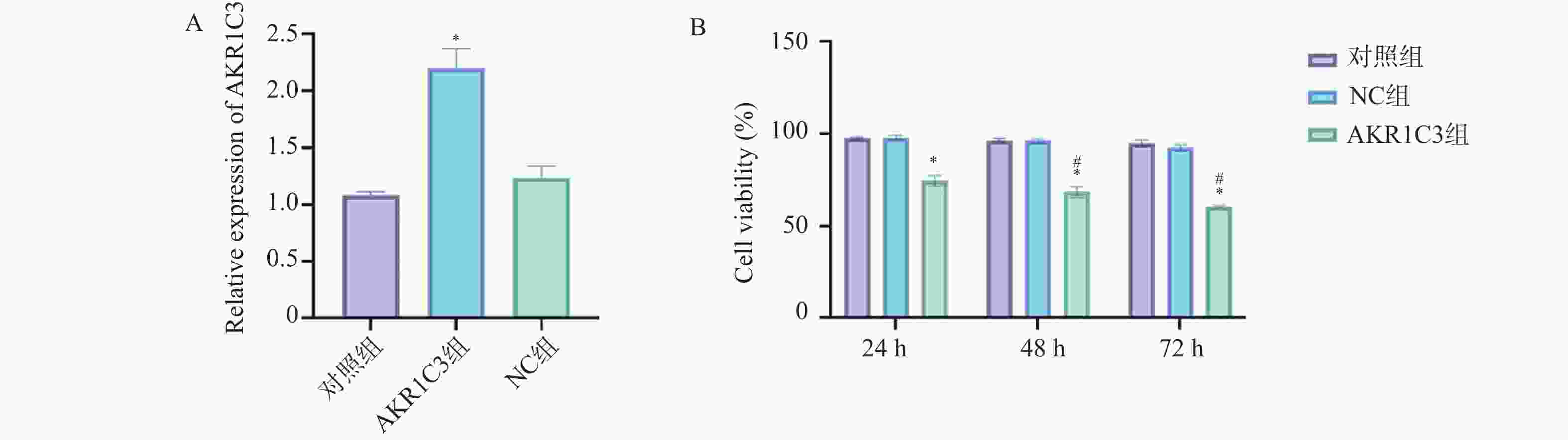
目的 探索酮还原酶家族1成员C3 (aldo-keto reductase family 1 member C3,AKR1C3)对乳腺癌恶性细胞生物学行为的干预作用及对程序性细胞死亡蛋白/程序性死亡-配体1(programmed cell death protein1/programmed death-ligand1,PD-1/PD-L)通路的影响。 方法 把MCF-7人乳腺癌细胞中NC组和AKR1C3组分别转染空质粒和AKR1C3质粒,采用MTT法检测转染后24 h、48 h、72 h细胞活力;采用流式细胞技术测定各组细胞的存活率以及早期、晚期凋亡比例;通过Transwell实验对各组细胞的迁移和侵袭能力进行检测;通过Western blot检测各组细胞PD-1、PD-L1、蛋白激酶B(protein kinase b,AKT)蛋白表达水平。使用C57BL/6小鼠构建荷瘤模型,将采用人乳腺癌MCF-7细胞转染NC质粒和AKR1C3质粒进行细胞荷瘤,每3 d测量瘤体积,持续21 d,绘制两组小鼠肿瘤生长曲线,并于实验终点测量肿瘤质量。 结果 相较于NC组,AKR1C3组细胞活力降低(P < 0.05),并且具有时间依赖效应(P < 0.05),迁移和侵袭能力降低(P < 0.05),早期凋亡和晚期凋亡比例升高(P < 0.05),PD-1、PD-L1、AKT蛋白表达水平降低(P < 0.05)。动物实验表明,AKR1C3组小鼠肿瘤体积降低,肿瘤质量下降(P < 0.05)。 结论 AKR1C3可以抑制人乳腺癌细胞恶性生物学行为,抑制PD-1/PD-L1信号通路蛋白表达。 Abstract:Objective To investigate the effect of aldo-keto reductase family 1 member C3(AKR1C3) on malignant biological behavior of breast cancer cells and its influence on the programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) pathway. Methods MCF-7 human breast cancer cells with NC and AKR1C3 groups transfected with NC plasmid and AKR1C3 plasmid respectively. Cell viability at 24 h/48 h/72 h post-transfection was assessed by MTT assay; flow cytometry measured cell survival rate and early/late apoptosis ratios; Transwell evaluated migration and invasion capabilities; western blot detected PD-1, PD-L1, protein kinase B (AKT) protein expression. For in vivo experiments, the C57BL/6 mice were used to establish tumor-bearing models. Human breast cancer MCF-7 cells transfected with NC plasmid and AKR1C3 plasmid were used for cell tumor-bearing. The tumor volume was measured every 3 days for 21 days, draw the tumor growth curves of the two groups of mice and measure the tumor mass at the end of the experiment. Results Compared to NC groups, the AKR1C3 group showed significantly reduced cell viability (time-dependent) (P < 0.05), suppressed migration/invasion (P < 0.05), increased early/late apoptosis ratios (P < 0.05), and downregulated PD-1/PD-L1/AKT protein expression (P < 0.05). In vivo, AKR1C3 group exhibited reduced tumor volume and weight (P < 0.05). Conclusion AKR1C3 inhibits malignant biological behaviors in breast cancer cells and suppresses PD-1/PD-L1 signaling pathway protein expression. -
Key words:
- AKR1C3 /
- Human breast cancer /
- Apoptosis /
- Migration /
- PD-1 / PD-L1 pathway /
- Invasion /
- AKT /
- PPR5
-
表 1 AKR1C3引物序列信息
Table 1. AKR1C3 primer sequence information
基因 引物序列 引物长度(bp) AKR1C3 F 5′-CAA GCG TCC TAA TTG TGG TCA-3′
R 5′-CCC TGC TAG ATG TCC AAC TGA TT-3′22 GAPDH F 5′-AGA AGG CTG GGG CTC ATT TG-3′
R 5′ AGG GGC CAT CCA CAG TCT TC-3′20 表 2 过表达AKR1C3对人乳腺癌MCF-7细胞凋亡的影响($\bar x \pm s $,n = 3)
Table 2. Effect of AKR1C3 overexpression on apoptosis in human breast cancer MCF-7 cells ($\bar x \pm s $,n = 3)
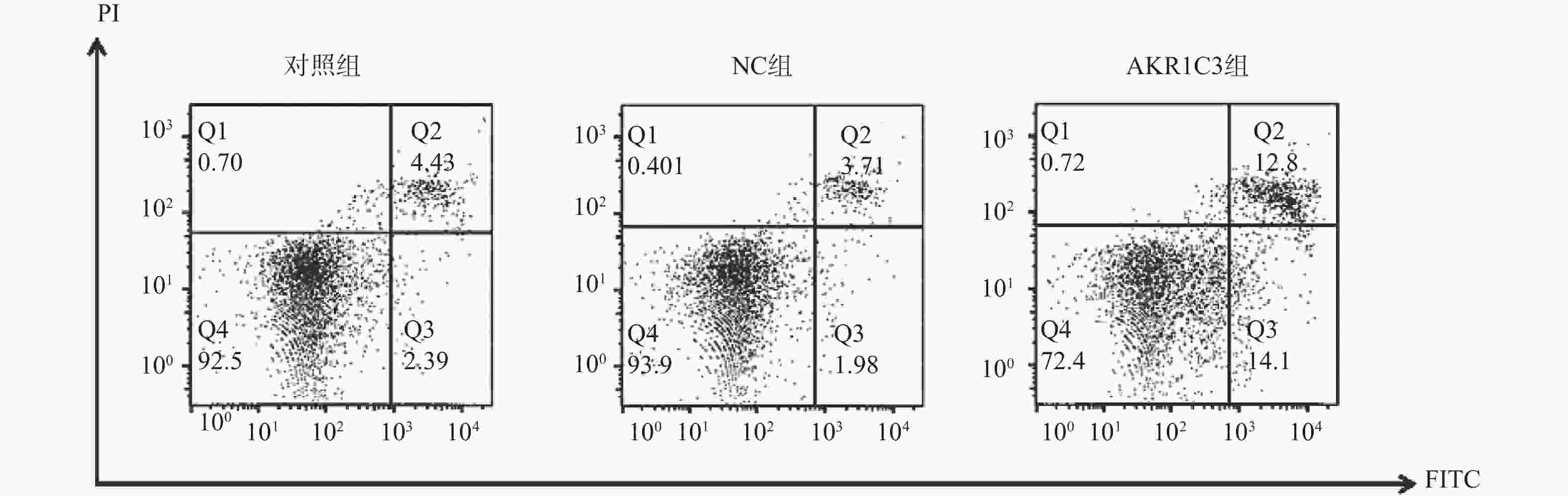
组别 细胞存活(%) 早期凋亡(%) 晚期凋亡(%) 对照组 94.51 ± 0.91 3.21 ± 0.18 1.06 ± 0.05 NC组 93.21 ± 0.98 3.16 ± 0.47 1.28 ± 0.52 AKR1C3组 61.71 ± 5.73* 13.21 ± 0.41* 25.21 ± 1.02* F 89.675 715.490 1320.242 P <0.001* <0.001* <0.001* 与NC组比较,*P < 0.05。 -
[1] 张作伟, 吴玉戈, 李贵轩. 乳腺癌组织及细胞系中CCDC34的表达及其生物学功能[J]. 解剖科学进展, 2022, 28(1): 35-38. [2] De Rose F, Meduri B, De Santis M C, et al. Rethinking breast cancer follow-up based on individual risk and recurrence management[J]. Cancer Treatment Reviews, 2022, 109: 102434. doi: 10.1016/j.ctrv.2022.102434 [3] Zhang M, Bai X, Zeng X, et al. circRNA-miRNA-mRNA in breast cancer[J]. Clinica Chimica Acta, 2021, 523: 120-130. doi: 10.1016/j.cca.2021.09.013 [4] Rios A C, Jacco V R, Scheele C L. Multidimensional Imaging of Breast Cancer[J]. Cold Spring Harbor Perspectives in Medicine, 2023, 13(5): a041330. doi: 10.1101/cshperspect.a041330 [5] Jokar N, Velikyan I, Ahmadzadehfar H, et al. Theranostic approach in breast cancer: A treasured tailor for future oncology[J]. Clinical Nuclear Medicine, 2021, 46(8): e410-e420. doi: 10.1097/RLU.0000000000003678 [6] 叶思婷. AKR1C3免疫组化表达与肿瘤相关性的Meta分析[D]. 福州: 福建医科大学, 2020. [7] Penning T M, Burczynski M E, Jez J M, et al. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: Functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones [J]. Biochemical Journal, 2021, 351(Pt 1): 67-77. [8] Zhang Z, Qiu X, Yan Y, et al. Evaluation of ferroptosis-related gene AKR1C1 as a novel biomarker associated with the immune microenvironment and prognosis in breast cancer[J]. International Journal of General Medicine, 2021, 14: 6189-6200. doi: 10.2147/IJGM.S329031 [9] Lee D G, Schuetz J M, Lai A S, et al. Interactions between exposure to polycyclic aromatic hydrocarbons and xenobiotic metabolism genes, and risk of breast cancer[J]. Breast Cancer, 2022, 29(1): 38-49. doi: 10.1007/s12282-021-01279-0 [10] Vranic S, Cyprian F S, Gatalica Z, et al. PD-L1 status in breast cancer: Current view and perspectives[J]. Seminars in Cancer Biology, 2021, 72: 146-154. doi: 10.1016/j.semcancer.2019.12.003 [11] Wu M, Huang Q, Xie Y, et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation[J]. Journal of Hematology & Oncology, 2022, 15(1): 24. [12] Hu X, Wang J, Chu M, et al. Emerging role of ubiquitination in the regulation of pd-1/pd-l1 in cancer immunotherapy[J]. Molecular Therapy, 2021, 29(3): 908-919. doi: 10.1016/j.ymthe.2020.12.032 [13] 麻艺群, 汤諹. PI3K/AKT信号通路及AKR1C3在人瘢痕疙瘩形成中的作用机制研究[J]. 中华整形外科杂志, 2022, 38(1): 83-92. [14] 魏瑜, 李志芳, 曹雷雨, 等. 敲减辅酶Q10B表达对裸鼠食管鳞癌皮下移植瘤细胞增殖、凋亡及上皮间质转化的影响[J]. 山东医药, 2024, 64(28): 40-43. [15] Wang S, Xiong Y, Zhang Q, et al. Clinical significance and immunogenomic landscape analyses of the immune cell signature based prognostic model for patients with breast cancer[J]. Briefings in Bioinformatics, 2021, 22(4): bbaa311. doi: 10.1093/bib/bbaa311 [16] Zhang Y N, Xia K R, Li C Y, et al. Review of breast cancer pathologigcal image processing[J]. BioMed Research International, 2021, 2021: 1994764. doi: 10.1155/2021/1994764 [17] Liang Y, Zhang H, Song X, et al. Metastatic heterogeneity of breast cancer: molecular mechanism and potential therapeutic targets[J]. Seminars in Cancer Biology, 2020, 60: 14-27. doi: 10.1016/j.semcancer.2019.08.012 [18] Meng F, Li WF, Jung D, et al. A novel selective AKR1C3-activated prodrug AST-3424/OBI-3424 exhibits broad anti-tumor activity[J]. American Journal of Cancer Research, 2021, 11(7): 3645-3659. [19] Liu H, Gao L, Xie T, et al. Identification and validation of a prognostic signature for prostate cancer based on ferroptosis-related genes[J]. Frontiers in Oncology, 2021, 11: 623313. doi: 10.3389/fonc.2021.623313 [20] Toscan C E, McCalmont H, Ashoorzadeh A, et al. The third generation AKR1C3-activated prodrug, ACHM-025, eradicates disease in preclinical models of aggressive T-cell acute lymphoblastic leukemia[J]. Blood Cancer Journal, 2024, 14(1): 192. doi: 10.1038/s41408-024-01180-x [21] Zhou Q, Tian W, Jiang Z, et al. A positive feedback loop of AKR1C3-mediated activation of NF-κB and STAT3 facilitates proliferation and metastasis in hepatocellular carcinoma[J]. Cancer Research, 2021, 81(5): 1361-1374. doi: 10.1158/0008-5472.CAN-20-2480 [22] Lee O, Fought A J, Shidfar A, et al. Association of genetic polymorphisms with local steroid metabolism in human benign breasts[J]. Steroids, 2022, 177: 108937. doi: 10.1016/j.steroids.2021.108937 [23] Vogeley C, Sondermann N C, Woeste S, et al. Unraveling the differential impact of PAHs and dioxin-like compounds on AKR1C3 reveals the EGFR extracellular domain as a critical determinant of the AHR response[J]. Environment International, 2022, 158: 106989. doi: 10.1016/j.envint.2021.106989 [24] Verma K, Gupta N, Zang T, et al. AKR1C3 inhibitor kv-37 exhibits antineoplastic effects and potentiates enzalutamide in combination therapy in prostate adenocarcinoma cells[J]. Molecular Cancer Therapeutics, 2021, 17(9): 1833-1845. [25] 朱鹏飞. AKR1C3促进肝癌细胞增殖、侵袭和转移的机制研究[D]. 郑州: 郑州大学, 2022. [26] Voabil P, de Bruijn M, Roelofsen L M, et al. An ex vivo tumor fragment platform to dissect response to PD-1 blockade in cancer[J]. Nature Medicine, 2021, 27(7): 1250-1261. doi: 10.1038/s41591-021-01398-3 [27] Munari E, Mariotti F R, Quatrini L, et al. PD-1/PD-L1 in cancer: pathophysiological, diagnostic and therapeutic aspects[J]. International Journal of Molecular Sciences, 2021, 22(10): 5123. doi: 10.3390/ijms22105123 [28] Sugiura D, Okazaki I M, Maeda T K, et al. PD-1 agonism by anti-CD80 inhibits T cell activation and alleviates autoimmunity[J]. Nature Immunology, 2022, 23(3): 399-410. doi: 10.1038/s41590-021-01125-7 [29] Helou D G, Quach C, Fung M, et al. Human PD-1 agonist treatment alleviates neutrophilic asthma by reprogramming T cells[J]. Journal of Allergy and Clinical Immunology, 2023, 151(2): 526-538. e8. [30] Ge Z, Zhou G, Lucia C C, et al. TIGIT and PD1 co-blockade restores ex vivo functions of human tumor-infiltrating CD8+ T cells in hepatocellular carcinoma[J]. Cellular and Molecular Gastroenterology and Hepatology, 2021, 12(2): 443-464. doi: 10.1016/j.jcmgh.2021.03.003 [31] Ledys F, Kalfeist L, Galland L, et al. Therapeutic associations comprising anti-PD-1/PD-L1 in breast cancer: clinical challenges and perspectives[J]. Cancers, 2021, 13(23): 5999. doi: 10.3390/cancers13235999 [32] Bassez A, Vos H, Van D L, et al. A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer[J]. Nature Medicine, 2021, 27(5): 820-832. doi: 10.1038/s41591-021-01323-8 [33] Jin M, Fang J, Peng J, et al. PD-1/PD-L1 immune checkpoint blockade in breast cancer: research insights and sensitization strategies[J]. Molecular Cancer, 2024, 23(1): 266. doi: 10.1186/s12943-024-02176-8 [34] Song Y, Bugada L, Li R, et al. Albumin nanoparticle containing a PI3Kγ inhibitor and paclitaxel in combination with α-PD1 induces tumor remission of breast cancer in mice[J]. Science Translational Medicine, 2022, 14(643): eabl3649. doi: 10.1126/scitranslmed.abl3649 [35] Hammerl D, Martens J W M, Timmermans M, et al. Spatial immunophenotypes predict response to anti-PD1 treatment and capture distinct paths of T cell evasion in triple negative breast cancer[J]. Nature Communications, 2021, 12(1): 5668. doi: 10.1038/s41467-021-25962-0 -





 下载:
下载: