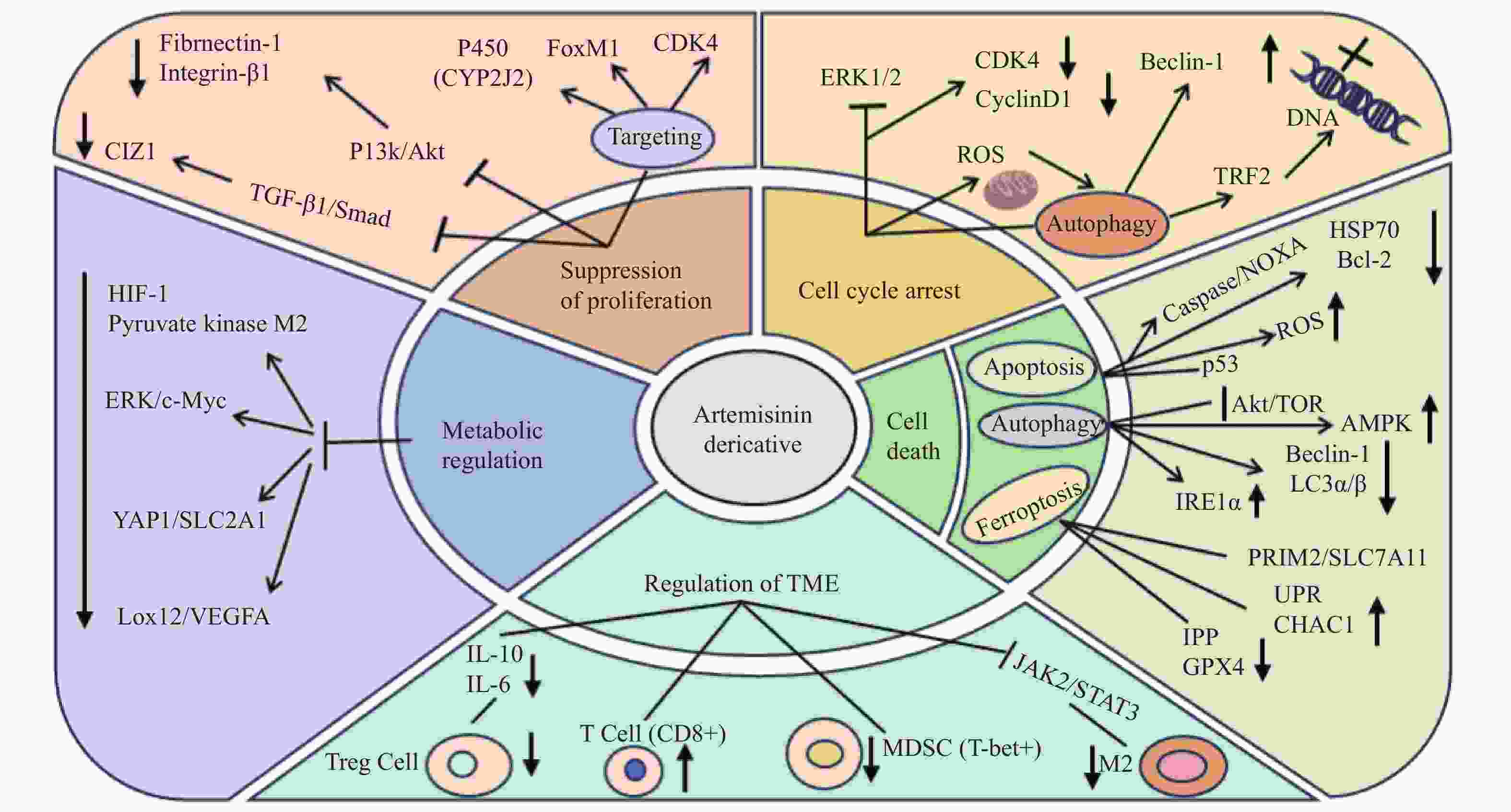
|
[1]
|
Ma L,Zhang M,Zhao R,et al. Plant natural products: Promising resources for cancer chemoprevention[J]. Molecules (Basel,Switzerland),2021,26(4):933-955.
|
|
[2]
|
Sonkin D,Thomas A,Teicher B A. Cancer treatments: Past,present,and future[J]. Cancer Genetics,2024,286-287(5):18-24.
|
|
[3]
|
Guo M,Jin J,Zhao D,et al. Research advances on anti-cancer natural products[J]. Frontiers in Oncology,2022,12(5):866154-866170.
|
|
[4]
|
Marchesi E,Perrone D,Navacchia M L. Molecular hybridization as a strategy for developing artemisinin-derived anticancer candidates[J]. Pharmaceutics,2023,15(9):2185-2224.
|
|
[5]
|
Zeng Z W,Chen D,Chen L,et al. A comprehensive overview of Artemisinin and its derivatives as anticancer agents[J]. European Journal of Medicinal Chemistry,2023,247(4):115000-115030.
|
|
[6]
|
Trimble C L,Levinson K,Maldonado L,et al. A first-in-human proof-of-concept trial of intravaginal artesunate to treat cervical intraepithelial neoplasia 2/3 (CIN2/3)[J]. Gynecologic Oncology,2020,157(1):188-194.
|
|
[7]
|
Ferrall L,Lin K Y,Roden R B S,et al. Cervical cancer immunotherapy: Facts and hopes[J]. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research,2021,27(18):4953-4973.
|
|
[8]
|
Dong J,Chen Y,Yang W,et al. Antitumor and anti-angiogenic effects of artemisinin on breast tumor xenografts in nude mice[J]. Research in Veterinary Science,2020,129(2):66-69.
|
|
[9]
|
Li Y,Zhou X,Liu J,et al. Dihydroartemisinin inhibits the tumorigenesis and metastasis of breast cancer via downregulating CIZ1 expression associated with TGF-β1 signaling[J]. Life Sciences,2020,248(9):117454-117463.
|
|
[10]
|
Zhang Q,Yi H,Yao H,et al. Artemisinin derivatives inhibit non-small cell lung cancer cells through induction of ROS-dependent apoptosis/ferroptosis[J]. Journal of Cancer,2021,12(13):4075-4085.
|
|
[11]
|
Zhang Y,Wang Y,Li Y,et al. Dihydroartemisinin and artesunate inhibit aerobic glycolysis via suppressing c-myc signaling in non-small cell lung cancer[J]. Biochemical Pharmacology,2022,198(4):114941-114949.
|
|
[12]
|
Xiao X,Li Y,Wang Y,et al. Dihydroartemisinin inhibits Lewis Lung carcinoma progression by inducing macrophages M1 polarization via AKT/mTOR pathway[J]. International Immunopharmacology,2022,103(2):108427-108429.
|
|
[13]
|
Jia J,Qin Y,Zhang L,et al. Artemisinin inhibits gallbladder cancer cell lines through triggering cell cycle arrest and apoptosis[J]. Molecular Medicine Reports,2016,13(5):4461-4468.
|
|
[14]
|
Kandoth C,McLellan M D,Vandin F,et al. Mutational landscape and significance across 12 major cancer types[J]. Nature,2013,502(7471):333-339.
|
|
[15]
|
Zielińska K A,Katanaev V L. Information theory: New look at oncogenic signaling pathways[J]. Trends in Cell Biology,2019,29(11):862-875.
|
|
[16]
|
Tin A S,Sundar S N,Tran K Q,et al. Antiproliferative effects of artemisinin on human breast cancer cells requires the downregulated expression of the E2F1 transcription factor and loss of E2F1-target cell cycle genes[J]. Anti-Cancer Drugs,2012,23(4):370-379.
|
|
[17]
|
Nandi D,Cheema P S,Singal A,et al. Artemisinin mediates its tumor-suppressive activity in hepatocellular carcinoma through targeted inhibition of FoxM1[J]. Frontiers in Oncology,2021,11(1):751271-751289.
|
|
[18]
|
Liang R,Chen W,Chen X Y,et al. Dihydroartemisinin inhibits the tumorigenesis and invasion of gastric cancer by regulating STAT1/KDR/MMP9 and P53/BCL2L1/CASP3/7 pathways[J]. Pathology,Research and Practice,2021,218(2):153318-153330.
|
|
[19]
|
Wu R,Gao Y,Wu J,et al. Semi-synthetic product dihydroartemisinin inhibited fibronectin-1 and integrin-β1 and interfered with the migration of HCCLM6 cells via PI3K-AKT pathway[J]. Biotechnology Letters,2020,42(6):917-926.
|
|
[20]
|
Zhu X,Yang M,Song Z,et al. Artemether inhibits proliferation,invasion and migration of hepatocellular carcinoma cells via targeting of CYP2J2[J]. Oncology Letters,2022,23(6):180-188.
|
|
[21]
|
Slezakova S,Ruda-Kucerova J. Anticancer activity of Artemisinin and its derivatives[J]. Anticancer Research,2017,37(11):5995-6003.
|
|
[22]
|
Willoughby J A,Sundar S N,Cheung M,et al. Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression[J]. Journal of Biological Chemistry,2009,284(4):2203-2213.
|
|
[23]
|
Ma Q,Liao H,Xu L,et al. Autophagy-dependent cell cycle arrest in esophageal cancer cells exposed to dihydroartemisinin[J]. Chinese Medicine,2020,15(9):37-49.
|
|
[24]
|
Li B,Bu S,Sun J,et al. Artemisinin derivatives inhibit epithelial ovarian cancer cells via autophagy-mediated cell cycle arrest[J]. Acta Biochimica et Biophysica Sinica,2018,50(12):1227-1235.
|
|
[25]
|
Greenshields A L,Shepherd T G,Hoskin D W. Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate[J]. Molecular Carcinogenesis,2017,56(1):75-93.
|
|
[26]
|
Xu C,Zhang H,Mu L,et al. Artemisinins as anticancer drugs: Novel therapeutic approaches,molecular mechanisms,and clinical trials[J]. Frontiers in Pharmacology,2020,11(10):529881-529895.
|
|
[27]
|
Eskandari E,Eaves C J. Paradoxical roles of caspase-3 in regulating cell survival,proliferation,and tumorigenesis[J]. Journal of Cell Biology,2022,221(6):e202201159-e202201172.
|
|
[28]
|
Efferth T,Rücker G,Falkenberg M,et al. Detection of apoptosis in KG-1a leukemic cells treated with investigational drugs[J]. Arzneimittel Forschung,1996,46(2):196-200.
|
|
[29]
|
Lang S J,Schmiech M,Hafner S,et al. Antitumor activity of an artemisia annua herbal preparation and identification of active ingredients[J]. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology,2019,62(12):152962-152971.
|
|
[30]
|
Noori S,Hassan Z M,Farsam V. Artemisinin as a Chinese medicine,selectively induces apoptosis in pancreatic tumor cell line[J]. Chinese Journal of Integrative Medicine,2014,20(8):618-623.
|
|
[31]
|
Mondal A,Chatterji U. Artemisinin represses telomerase subunits and induces apoptosis in HPV-39 infected human cervical cancer cells[J]. Journal of Cellular Biochemistry,2015,116(9):1968-1981.
|
|
[32]
|
Pirali M,Taheri M,Zarei S,et al. Artesunate,as a HSP70 ATPase activity inhibitor,induces apoptosis in breast cancer cells[J]. International Journal of Biological Macromolecules,2020,164(5):3369-3375.
|
|
[33]
|
Cabello C M,Lamore S D,Bair W B,et al. The redox antimalarial dihydroartemisinin targets human metastatic melanoma cells but not primary melanocytes with induction of NOXA-dependent apoptosis[J]. Investigational New Drugs,2012,30(4):1289-1301.
|
|
[34]
|
Chatterjee R,Shukla A,Chakrabarti K,et al. CLEC12A sensitizes differentially responsive breast cancer cells to the anti-cancer effects of artemisinin by repressing autophagy and inflammation[J]. Frontiers in Oncology,2023,13(12):1242432-1242449.
|
|
[35]
|
Huang Z,Gan S,Zhuang X,et al. Artesunate inhibits the cell growth in colorectal cancer by promoting ROS-dependent cell senescence and autophagy[J]. Cells,2022,11(16):2472-2492.
|
|
[36]
|
Zhou X,Chen Y,Wang F,et al. Artesunate induces autophagy dependent apoptosis through upregulating ROS and activating AMPK-mTOR-ULK1 axis in human bladder cancer cells[J]. Chemico-Biological Interactions,2020,331(17):109273-109284.
|
|
[37]
|
Chen X,He L Y,Lai S,et al. Dihydroartemisinin inhibits the migration of esophageal cancer cells by inducing autophagy[J]. Oncology Letters,2020,20(4):94-102.
|
|
[38]
|
Zhou Q,Meng Y,Li D,et al. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies[J]. Signal Transduction and Targeted Therapy,2024,9(1):55-84.
|
|
[39]
|
Chen F,Fan Y,Hou J,et al. Integrated analysis identifies TfR1 as a prognostic biomarker which correlates with immune infiltration in breast cancer[J]. Aging,2021,13(17):21671-21699.
|
|
[40]
|
Yuan B,Liao F,Shi Z Z,et al. Dihydroartemisinin inhibits the proliferation,colony formation and induces ferroptosis of lung cancer cells by inhibiting PRIM2/SLC7A11 axis[J]. OncoTargets and Therapy,2020,13(1):10829-10840.
|
|
[41]
|
Wang Z,Li M,Liu Y,et al. Dihydroartemisinin triggers ferroptosis in primary liver cancer cells by promoting and unfolded protein response-induced upregulation of CHAC1 expression[J]. Oncology Reports,2021,46(5):240-253.
|
|
[42]
|
Roh J L,Kim E H,Jang H,et al. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis[J]. Redox Biology,2017,11(1):254-262.
|
|
[43]
|
Liang L,Liu Y,Wu X,et al. Artesunate induces ferroptosis by inhibiting the nuclear localization of SREBP2 in myeloma cells[J]. International Journal of Medical Sciences,2023,20(12):1535-1550.
|
|
[44]
|
Hartupee C,Nagalo B M,Chabu C Y,et al. Pancreatic cancer tumor microenvironment is a major therapeutic barrier and target[J]. Frontiers in Immunology,2024,15(2):1287459-1287475.
|
|
[45]
|
Zhang Q,Sioud M. Tumor-associated macrophage subsets: Shaping polarization and targeting[J]. International Journal of Molecular Sciences,2023,24(8):7493-7524.
|
|
[46]
|
Yu R,Jin L,Li F,et al. Dihydroartemisinin inhibits melanoma by regulating CTL/treg anti-tumor immunity and STAT3-mediated apoptosis via IL-10 dependent manner[J]. Journal of Dermatological Science,2020,99(3):193-202.
|
|
[47]
|
Wang C Z,Wan C,Luo Y,et al. Effects of dihydroartemisinin,a metabolite of artemisinin,on colon cancer chemoprevention and adaptive immune regulation[J]. Molecular Biology Reports,2022,49(4):2695-2709.
|
|
[48]
|
Mancuso R I,Olalla Saad S T,Azambuja J H. Artesunate switches monocytes to an inflammatory phenotype with the ability to kill leukemic cells[J]. International Journal of Molecular Sciences,2021,22(2):608-621.
|
|
[49]
|
Cao Y,Feng Y H,Gao L W,et al. Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo[J]. International Immunopharmacology,2019,70(5):110-116.
|
|
[50]
|
Li Z,Munim M B,Sharygin D A,et al. Understanding the Warburg effect in cancer[J]. Cold Spring Harbor Perspectives in Medicine,2024,14(9):a041532.
|
|
[51]
|
Wong Y K,Xu C,Kalesh K A,et al. Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action[J]. Medicinal Research Reviews,2017,37(6):1492-1517.
|
|
[52]
|
Wang M,Chen H,He X,et al. Artemisinin inhibits the development of esophageal cancer by targeting HIF-1α to reduce glycolysis levels[J]. Journal of Gastrointestinal Oncology,2022,13(5):2144-2153.
|
|
[53]
|
Peng Q,Hao L,Guo Y,et al. Dihydroartemisinin inhibited the Warburg effect through YAP1/SLC2A1 pathway in hepatocellular carcinoma[J]. Journal of Natural Medicines,2023,77(1):28-40.
|
|
[54]
|
Ding X,Zhang Y,Liang J,et al. Dihydroartemisinin potentiates VEGFR-TKIs antitumorigenic effect on osteosarcoma by regulating Loxl2/VEGFA expression and lipid metabolism pathway[J]. Journal of Cancer,2023,14(5):809-820.
|





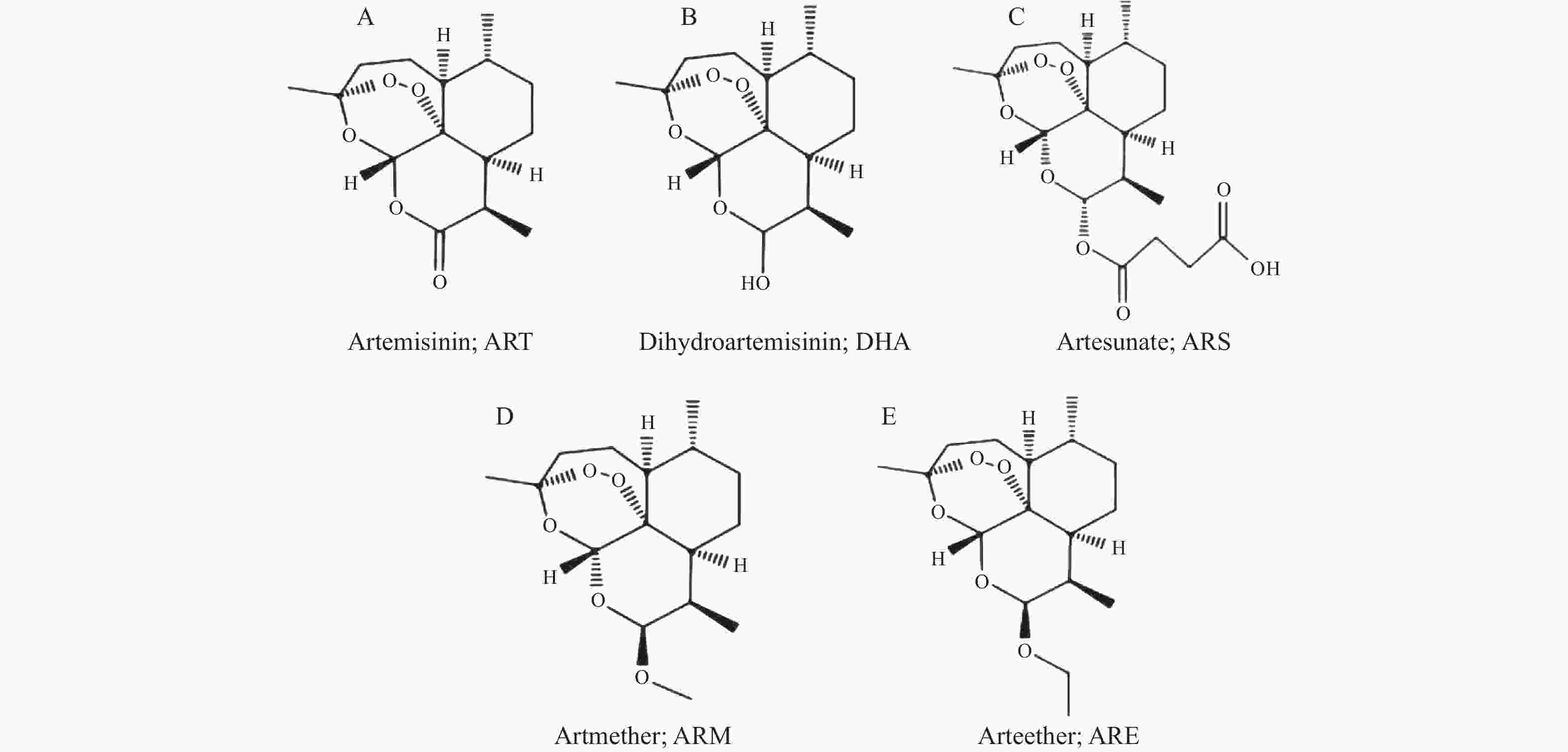
 下载:
下载: