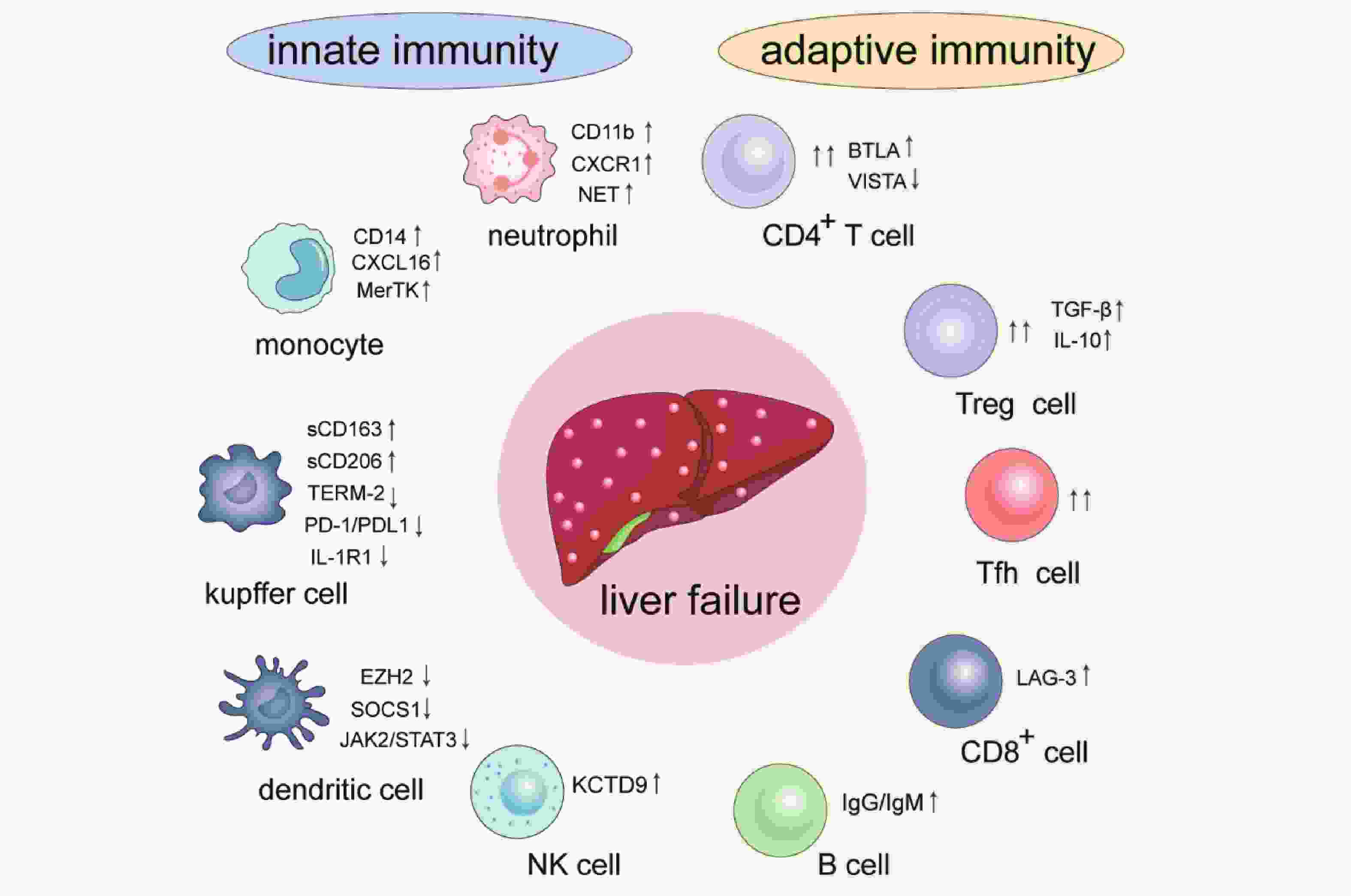
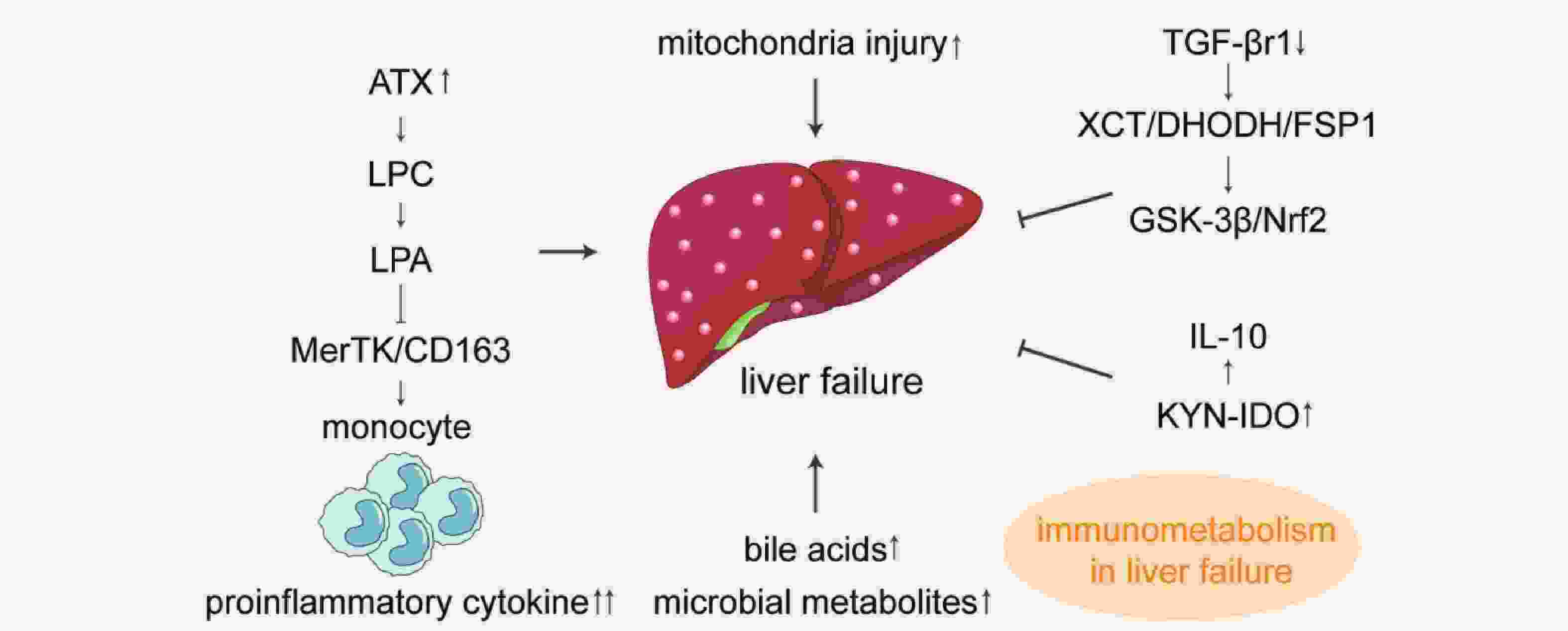
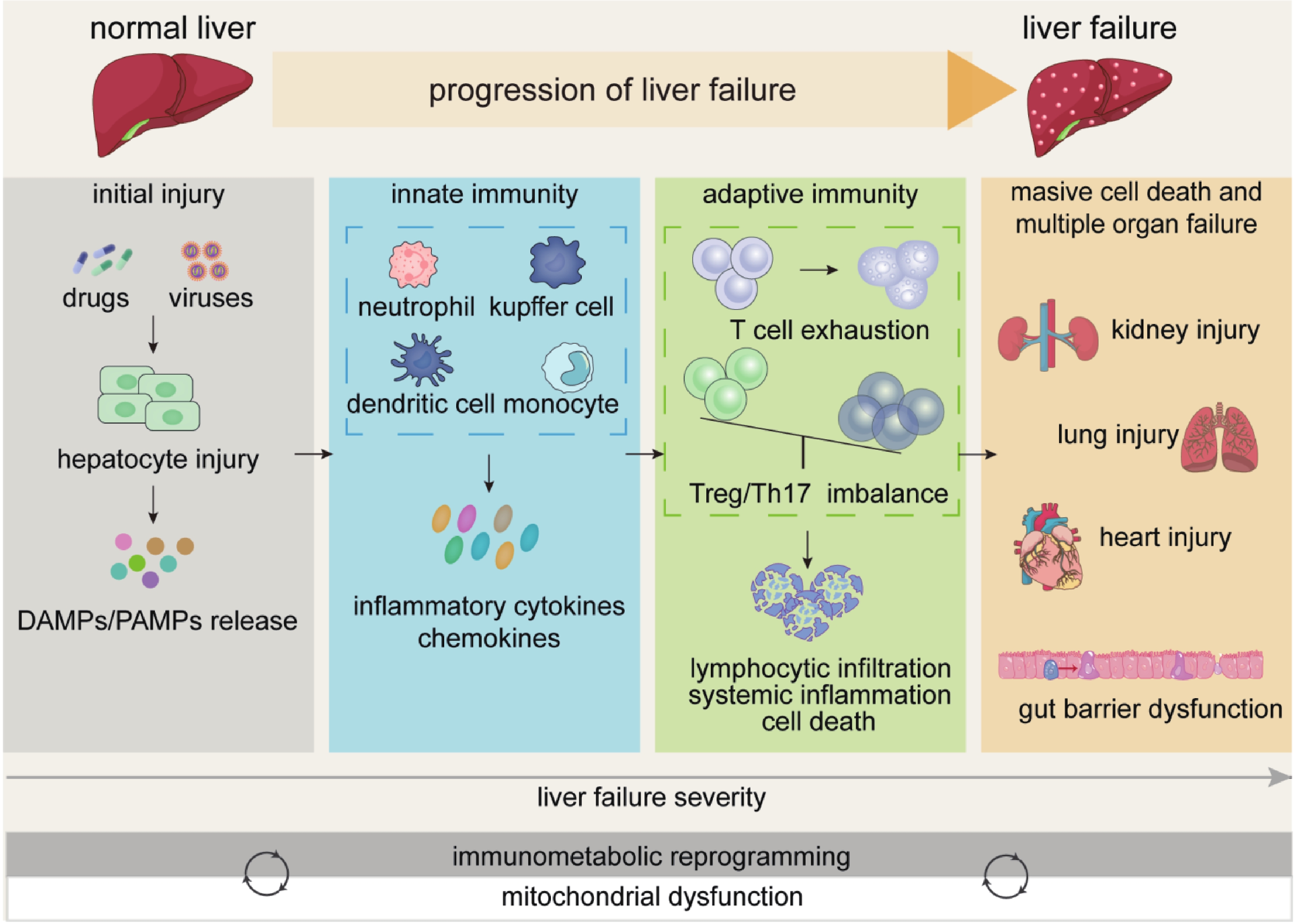
|
[1]
|
中华医学会肝病学分会重型肝病与人工肝学组, 中华医学会感染病学分会肝衰竭与人工肝学组. 肝衰竭诊治指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40(12): 2371-2387.
|
|
[2]
|
Mak L Y, Liu K, Chirapongsathorn S, et al. Liver diseases and hepatocellular carcinoma in the Asia-Pacific region: burden, trends, challenges and future directions[J]. Nat Rev Gastroenterol Hepatol, 2024, 21(12): 834-851. doi: 10.1038/s41575-024-00967-4
|
|
[3]
|
Devarbhavi H, Asrani S K, Arab J P, et al. Global burden of liver disease: 2023 update[J]. J Hepatol, 2023, 79(2): 516-537.
|
|
[4]
|
Hernaez R, Kramer J R, Liu Y, et al. Prevalence and short-term mortality of acute-on-chronic liver failure: A national cohort study from the USA[J]. J Hepatol, 2019, 70(4): 639-647. doi: 10.1016/j.jhep.2018.12.018
|
|
[5]
|
Khanam A, Kottilil S. Abnormal Innate Immunity in Acute-on-Chronic Liver Failure: Immunotargets for Therapeutics[J]. Front Immunol, 2020, 11: 2013. doi: 10.3389/fimmu.2020.02013
|
|
[6]
|
Ahmed O, Robinson M W, O'Farrelly C. Inflammatory processes in the liver: Divergent roles in homeostasis and pathology[J]. Cell Mol Immunol, 2021, 18(6): 1375-1386. doi: 10.1038/s41423-021-00639-2
|
|
[7]
|
Wen Y, Lambrecht J, Ju C, et al. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities[J]. Cell Mol Immunol, 2021, 18(1): 45-56. doi: 10.1038/s41423-020-00558-8
|
|
[8]
|
Siwicki M, Gort-Freitas N A, Messemaker M, et al. Resident kupffer cells and neutrophils drive liver toxicity in cancer immunotherapy[J]. Sci Immunol, 2021, 6(61): eabi7083. doi: 10.1126/sciimmunol.abi7083
|
|
[9]
|
Wu W, Sun S, Wang Y, et al. Circulating neutrophil dysfunction in HBV-related acute-on-chronic liver failure[J]. Front Immunol, 2021, 12: 620365. doi: 10.3389/fimmu.2021.620365
|
|
[10]
|
Liu K, Wang F S, Xu R. Neutrophils in liver diseases: pathogenesis and therapeutic targets[J]. Cell Mol Immunol, 2021, 18(1): 38-44. doi: 10.1038/s41423-020-00560-0
|
|
[11]
|
Chauhan A, Sheriff L, Hussain M T, et al. The platelet receptor CLEC-2 blocks neutrophil mediated hepatic recovery in acetaminophen induced acute liver failure[J]. Nat Commun, 2020, 11(1): 1939. doi: 10.1038/s41467-020-15584-3
|
|
[12]
|
Kolodziejczyk A A, Federici S, Zmora N, et al. Acute liver failure is regulated by MYC-and microbiome-dependent programs[J]. Nat Med, 2020, 26(12): 1899-1911. doi: 10.1038/s41591-020-1102-2
|
|
[13]
|
Meijenfeldt F A, Stravitz R T, Zhang J, et al. Generation of neutrophil extracellular traps in patients with acute liver failure is associated with poor outcome[J]. Hepatology, 2022, 75(3): 623-633. doi: 10.1002/hep.32174
|
|
[14]
|
Triantafyllou E, Pop O T, Possamai LA, et al. MerTK expressing hepatic macrophages promote the resolution of inflammation in acute liver failure[J]. Gut, 2018, 67(2): 333-347. doi: 10.1136/gutjnl-2016-313615
|
|
[15]
|
Albillos A, Hera Ad Ade L, Reyes E, et al. Tumour necrosis factor-alpha expression by activated monocytes and altered T-cell homeostasis in ascitic alcoholic cirrhosis: amelioration with norfloxacin[J]. J Hepatol, 2004, 40(4): 624-631. doi: 10.1016/j.jhep.2003.12.010
|
|
[16]
|
Wasmuth H E, Kunz D, Yagmur E, et al. Patients with acute on chronic liver failure display "sepsis-like" immune paralysis[J]. J Hepatol, 2005, 42(2): 195-201. doi: 10.1016/j.jhep.2004.10.019
|
|
[17]
|
Triantafyllou E, Woollard K J, McPhail M J W, et al. The role of monocytes and macrophages in acute and acute-on-chronic liver failure[J]. Front Immunol, 2018, 9: 2948. doi: 10.3389/fimmu.2018.02948
|
|
[18]
|
Bernsmeier C, Pop O T, Singanayagam A, et al. Patients with acute-on-chronic liver failure have increased numbers of regulatory immune cells expressing the receptor tyrosine kinase MERTK[J]. Gastroenterology, 2015, 148(3): 603-615. e614.
|
|
[19]
|
Leonhardt J, Haider R S, Sponholz C, et al. Circulating bile acids in liver failure activate TGR5 and induce monocyte dysfunction[J]. Cell Mol Gastroenterol Hepatol, 2021, 12(1): 25-40. doi: 10.1016/j.jcmgh.2021.01.011
|
|
[20]
|
Trovato F M, Zia R, Napoli S, et al. Dysregulation of the lysophosphatidylcholine/autotaxin/lysophosphatidic acid axis in acute-on-chronic liver failure is associated with mortality and systemic inflammation by lysophosphatidic acid-dependent monocyte activation[J]. Hepatology, 2021, 74(2): 907-925. doi: 10.1002/hep.31738
|
|
[21]
|
Korf H, du Plessis J, van Pelt J, et al. Inhibition of glutamine synthetase in monocytes from patients with acute-on-chronic liver failure resuscitates their antibacterial and inflammatory capacity[J]. Gut, 2019, 68(10): 1872-1883. doi: 10.1136/gutjnl-2018-316888
|
|
[22]
|
Singanayagam A, Triantafyllou E. Macrophages in Chronic Liver Failure: Diversity, Plasticity and Therapeutic Targeting[J]. Front Immunol, 2021, 12: 661182. doi: 10.3389/fimmu.2021.661182
|
|
[23]
|
Nielsen M C, Hvidbjerg Gantzel R, Clària J, et al. Macrophage activation markers, CD163 and CD206, in acute-on-chronic liver failure[J]. Cells, 2020, 9(5).
|
|
[24]
|
Perugorria M J, Esparza-Baquer A, Oakley F, et al. Non-parenchymal TREM-2 protects the liver from immune-mediated hepatocellular damage[J]. Gut, 2019, 68(3): 533-546. doi: 10.1136/gutjnl-2017-314107
|
|
[25]
|
Triantafyllou E, Gudd C L, Mawhin M A, et al. PD-1 blockade improves kupffer cell bacterial clearance in acute liver injury[J]. J Clin Invest, 2021, 131(4).
|
|
[26]
|
Bai L, Kong M, Duan Z, et al. M2-like macrophages exert hepatoprotection in acute-on-chronic liver failure through inhibiting necroptosis-S100A9-necroinflammation axis[J]. Cell Death Dis, 2021, 12(1): 93. doi: 10.1038/s41419-020-03378-w
|
|
[27]
|
Zhang S, Jiang L, Hu H, et al. Pretreatment of exosomes derived from hUCMSCs with TNF-α ameliorates acute liver failure by inhibiting the activation of NLRP3 in macrophage[J]. Life Sci, 2020, 246: 117401. doi: 10.1016/j.lfs.2020.117401
|
|
[28]
|
Yu X, Zhou L, Deng Q, et al. rhIL-1Ra reduces hepatocellular apoptosis in mice with acute liver failure mainly by inhibiting the activities of kupffer cells[J]. Eur J Pharmacol, 2019, 854: 338-346. doi: 10.1016/j.ejphar.2019.03.031
|
|
[29]
|
Wang J, Liu Y, Ding H, et al. Mesenchymal stem cell-secreted prostaglandin E(2) ameliorates acute liver failure via attenuation of cell death and regulation of macrophage polarization[J]. Stem Cell Res Ther, 2021, 12(1): 15. doi: 10.1186/s13287-020-02070-2
|
|
[30]
|
Yu H, Yang W, Huang J, et al. GPR120 induces regulatory dendritic cells by inhibiting HK2-dependent glycolysis to alleviate fulminant hepatic failure[J]. Cell Death Dis, 2021, 13(1): 1. doi: 10.1038/s41419-021-04394-0
|
|
[31]
|
Wang Y, Wang Q, Wang B, et al. Inhibition of EZH2 ameliorates bacteria-induced liver injury by repressing RUNX1 in dendritic cells[J]. Cell Death Dis, 2020, 11(11): 1024. doi: 10.1038/s41419-020-03219-w
|
|
[32]
|
Li SS, Yang M, Chen YP, et al. Dendritic cells with increased expression of suppressor of cytokine signaling 1(SOCS1) gene ameliorate lipopolysaccharide/d-galactosamine-induced acute liver failure[J]. Mol Immunol, 2018, 101: 10-18. doi: 10.1016/j.molimm.2018.05.016
|
|
[33]
|
Chen Y, Hou C, Yang N, et al. Regulatory Effect of JAK2/STAT3 on the immune function of endotoxin-tolerant dendritic cells and its involvement in acute liver failure[J]. J Clin Transl Hepatol, 2022, 10(5): 879-890. doi: 10.14218/JCTH.2021.00175
|
|
[34]
|
Li H, Zhai N, Wang Z, et al. Regulatory NK cells mediated between immunosuppressive monocytes and dysfunctional T cells in chronic HBV infection[J]. Gut, 2018, 67(11): 2035-2044. doi: 10.1136/gutjnl-2017-314098
|
|
[35]
|
Chen T, Zhu L, Zhou Y, et al. KCTD9 contributes to liver injury through NK cell activation during hepatitis B virus-induced acute-on-chronic liver failure[J]. Clin Immunol, 2013, 146(3): 207-216. doi: 10.1016/j.clim.2012.12.013
|
|
[36]
|
Zhang X, Zhu L, Zhou Y, et al. Interference with KCTD9 inhibits NK cell activation and ameliorates fulminant liver failure in mice[J]. BMC Immunol, 2018, 19(1): 20. doi: 10.1186/s12865-018-0256-x
|
|
[37]
|
Taru V, Szabo G, Mehal W, et al. Inflammasomes in chronic liver disease: Hepatic injury, fibrosis progression and systemic inflammation[J]. J Hepatol, 2024, 81(5): 895-910. doi: 10.1016/j.jhep.2024.06.016
|
|
[38]
|
Huang S, Wang Y, Xie S, et al. Hepatic TGFβr1 deficiency attenuates lipopolysaccharide/D-galactosamine-induced acute liver failure through inhibiting GSK3β-Nrf2-Mediated hepatocyte apoptosis and ferroptosis[J]. Cell Mol Gastroenterol Hepatol, 2022, 13(6): 1649-1672. doi: 10.1016/j.jcmgh.2022.02.009
|
|
[39]
|
Xiang X, Feng D, Hwang S, et al. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice[J]. J Hepatol, 2020, 72(4): 736-745. doi: 10.1016/j.jhep.2019.11.013
|
|
[40]
|
Zhou T, Sun Y, Li M, et al. Enhancer of zeste homolog 2-catalysed H3K27 trimethylation plays a key role in acute-on-chronic liver failure via TNF-mediated pathway[J]. Cell Death Dis, 2018, 9(6): 590. doi: 10.1038/s41419-018-0670-2
|
|
[41]
|
Fu B, Yin S, Lin X, et al. PTPN14 aggravates inflammation through promoting proteasomal degradation of SOCS7 in acute liver failure[J]. Cell Death Dis, 2020, 11(9): 803. doi: 10.1038/s41419-020-03014-7
|
|
[42]
|
Du X X, Shi Y, Yang Y, et al. DAMP molecular IL-33 augments monocytic inflammatory storm in hepatitis B-precipitated acute-on-chronic liver failure[J]. Liver Int, 2018, 38(2): 229-238. doi: 10.1111/liv.13503
|
|
[43]
|
Khanam A, Trehanpati N, Sarin S K. Increased interleukin-23 receptor (IL-23R) expression is associated with disease severity in acute-on-chronic liver failure[J]. Liver Int, 2019, 39(6): 1062-1070. doi: 10.1111/liv.14015
|
|
[44]
|
Zaccherini G, Weiss E, Moreau R. Acute-on-chronic liver failure: Definitions, pathophysiology and principles of treatment[J]. JHEP Rep, 2021, 3(1): 100176. doi: 10.1016/j.jhepr.2020.100176
|
|
[45]
|
Moreau R. The pathogenesis of ACLF: The inflammatory response and immune function[J]. Semin Liver Dis, 2016, 36(2): 133-140. doi: 10.1055/s-0036-1583199
|
|
[46]
|
Moreau R, Clària J, Aguilar F, et al. Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF[J]. J Hepatol, 2020, 72(4): 688-701. doi: 10.1016/j.jhep.2019.11.009
|
|
[47]
|
Filliol A, Piquet-Pellorce C, Raguénès-Nicol C, et al. RIPK1 protects hepatocytes from kupffer cells-mediated TNF-induced apoptosis in mouse models of PAMP-induced hepatitis[J]. J Hepatol, 2017, 66(6): 1205-1213. doi: 10.1016/j.jhep.2017.01.005
|
|
[48]
|
Yan Y Y, Lin S, Zhu Y Y. Damage-associated molecular patterns and liver failure[J]. Zhonghua Gan Zang Bing Za Zhi, 2016, 24(8): 636-640.
|
|
[49]
|
Wu J, Han M, Li J, et al. Pattern recognition receptors and liver failure[J]. Crit Rev Immunol, 2019, 39(4): 289-311. doi: 10.1615/CritRevImmunol.2019031012
|
|
[50]
|
Qiang R, Liu X Z, Xu JC. The immune pathogenesis of acute-on-chronic liver failure and the danger hypothesis[J]. Front Immunol, 2022, 13: 935160. doi: 10.3389/fimmu.2022.935160
|
|
[51]
|
Ma M, Jiang W, Zhou R. DAMPs and DAMP-sensing receptors in inflammation and diseases[J]. Immunity, 2024, 57(4): 752-771. doi: 10.1016/j.immuni.2024.03.002
|
|
[52]
|
Fan X G, Pei S Y, Zhou D, et al. Melittin ameliorates inflammation in mouse acute liver failure via inhibition of PKM2-mediated Warburg effect[J]. Acta Pharmacol Sin, 2021, 42(8): 1256-1266. doi: 10.1038/s41401-020-00516-0
|
|
[53]
|
Rueschenbaum S, Ciesek S, Queck A, et al. Dysregulated adaptive immunity is an early event in liver cirrhosis preceding acute-on-chronic liver failure[J]. Front Immunol, 2020, 11: 534731.
|
|
[54]
|
Khamri W, Abeles R D, Hou TZ, et al. Increased expression of cytotoxic T-lymphocyte-associated protein 4 by T cells, Induced by B7 in sera, reduces adaptive immunity in patients with acute liver failure[J]. Gastroenterology, 2017, 153(1): 263-276. e268. doi: 10.1053/j.gastro.2017.03.023
|
|
[55]
|
Wang F, Sun W, Xiao Q, et al. Peripheral T lymphocytes predict the severity and prognosis in patients with HBV-related acute-on-chronic liver failure[J]. Medicine, 2021, 100(5): e24075. doi: 10.1097/MD.0000000000024075
|
|
[56]
|
Du B, Teng J, Yin R, et al. Increased circulating T follicular helper cells induced via IL-12/21 in patients with acute on chronic hepatitis B liver failure[J]. Front Immunol, 2021, 12: 641362. doi: 10.3389/fimmu.2021.641362
|
|
[57]
|
Yu X, Yang F, Shen Z, et al. BTLA contributes to acute-on-chronic liver failure infection and mortality through CD4+ T-cell exhaustion[J]. Nat Commun, 2024, 15(1): 1835. doi: 10.1038/s41467-024-46047-8
|
|
[58]
|
Yao J, Ji Y, Liu T, et al. Single-cell RNA sequencing shows T-cell exhaustion landscape in the peripheral blood of patients with hepatitis B virus-associated acute-on-chronic liver failure[J]. Gut Liver, 2024, 18(3): 520-530. doi: 10.5009/gnl220449
|
|
[59]
|
Zhou X, Li Y, Ji Y, et al. PD-1 Involvement in peripheral blood CD8+ T lymphocyte dysfunction in patients with acute-on-chronic liver failure[J]. J Clin Transl Hepatol, 2021, 9(3): 283-290.
|
|
[60]
|
Tang L, Wang X, Zhao R, et al. Yi-Qi-Jian-Pi formula ameliorates immune function in acute-on-chronic liver failure by upregulating autophagy and mitochondrial biogenesis in CD8+ T lymphocytes[J]. J Ethnopharmacol, 2023, 308: 116276. doi: 10.1016/j.jep.2023.116276
|
|
[61]
|
Shen C, Yan W Z, Zhao CY, et al. Increased CD4+CD25+ regulatory T cells correlate with poor short-term outcomes in hepatitis B virus-related acute-on-chronic liver failure patients[J]. J Microbiol Immunol Infect, 2015, 48(2): 137-146. doi: 10.1016/j.jmii.2013.11.001
|
|
[62]
|
Tan N H, Chen B, Peng J, et al. Treg/Th17 cell balance in patients with hepatitis B virus-related acute-on-chronic liver failure at different disease stages[J]. Biomed Res Int, 2021, 2021: 9140602. doi: 10.1155/2021/9140602
|
|
[63]
|
Zhang Y, Zhang X, Han J, et al. Downregulated VISTA enhances Th17 differentiation and aggravates inflammation in patients with acute-on-chronic liver failure[J]. Hepatol Int, 2023, 17(4): 1000-1015. doi: 10.1007/s12072-023-10505-0
|
|
[64]
|
Wang X, Zhong Y, Zhang R, et al. Wenyang huazhuo tuihuang formula inhibits the Th17/Treg cell Imbalance and protects against acute-on-chronic liver failure[J]. Evid Based Complement Alternat Med, 2022, 2022: 5652172.
|
|
[65]
|
Chen L, Huang Y, Chen Y, et al. Resolvin D1 promotes the resolution of inflammation in the ACLF rat model by increasing the proportion of Treg cells[J]. Immun Inflamm Dis, 2023, 11(11): e1076. doi: 10.1002/iid3.1076
|
|
[66]
|
Farci P, Diaz G, Chen Z, et al. B cell gene signature with massive intrahepatic production of antibodies to hepatitis B core antigen in hepatitis B virus-associated acute liver failure[J]. Proc Natl Acad Sci U S A, 2010, 107(19): 8766-8771. doi: 10.1073/pnas.1003854107
|
|
[67]
|
Perez-Andres M, Paiva B, Nieto WG, et al. Human peripheral blood B-cell compartments: a crossroad in B-cell traffic[J]. Cytometry B Clin Cytom, 2010, 78(Suppl 1): S47-60.
|
|
[68]
|
Martin V G, Wu Y B, Townsend C L, et al. Transitional B cells in early human B cell development-time to revisit the paradigm?[J]. Front Immunol, 2016, 7: 546.
|
|
[69]
|
Cardoso C C, Matiollo C, Pereira C H J, et al. B-cell compartment abnormalities are associated with ACLF and mortality in patients with liver cirrhosis[J]. Clin Res Hepatol Gastroenterol, 2021, 45(4): 101698. doi: 10.1016/j.clinre.2021.101698
|
|
[70]
|
Makowski L, Chaib M, Rathmell J C. Immunometabolism: from basic mechanisms to translation[J]. Immunol Rev, 2020, 295(1): 5-14. doi: 10.1111/imr.12858
|
|
[71]
|
Zhang I W, Curto A, López-Vicario C, et al. Mitochondrial dysfunction governs immunometabolism in leukocytes of patients with acute-on-chronic liver failure[J]. J Hepatol, 2022, 76(1): 93-106. doi: 10.1016/j.jhep.2021.08.009
|
|
[72]
|
Yu Z, Li J, Ren Z, et al. Switching from fatty acid oxidation to glycolysis improves the outcome of acute-on-chronic liver failure[J]. Adv Sci(Weinh), 2020, 7(7): 1902996.
|
|
[73]
|
Clària J, Moreau R, Fenaille F, et al. Orchestration of tryptophan-kynurenine pathway, Acute decompensation, and acute-on-chronic liver failure in cirrhosis[J]. Hepatology, 2019, 69(4): 1686-1701. doi: 10.1002/hep.30363
|
|
[74]
|
Park S H, Kwak J A, Jung S H, et al. Piperidylmethyloxychalcone improves immune-mediated acute liver failure via inhibiting TAK1 activity[J]. Exp Mol Med, 2017, 49(11): e392. doi: 10.1038/emm.2017.156
|
|
[75]
|
Bajaj J S, Reddy K R, O'Leary J G, et al. Serum levels of metabolites produced by intestinal microbes and lipid moieties independently associated with acute-on-chronic liver failure and death in patients with cirrhosis[J]. Gastroenterology, 2020, 159(5): 1715-1730. e1712. doi: 10.1053/j.gastro.2020.07.019
|
|
[76]
|
Li J, Liang X, Jiang J, et al. PBMC transcriptomics identifies immune-metabolism disorder during the development of HBV-ACLF[J]. Gut, 2022, 71(1): 163-175. doi: 10.1136/gutjnl-2020-323395
|





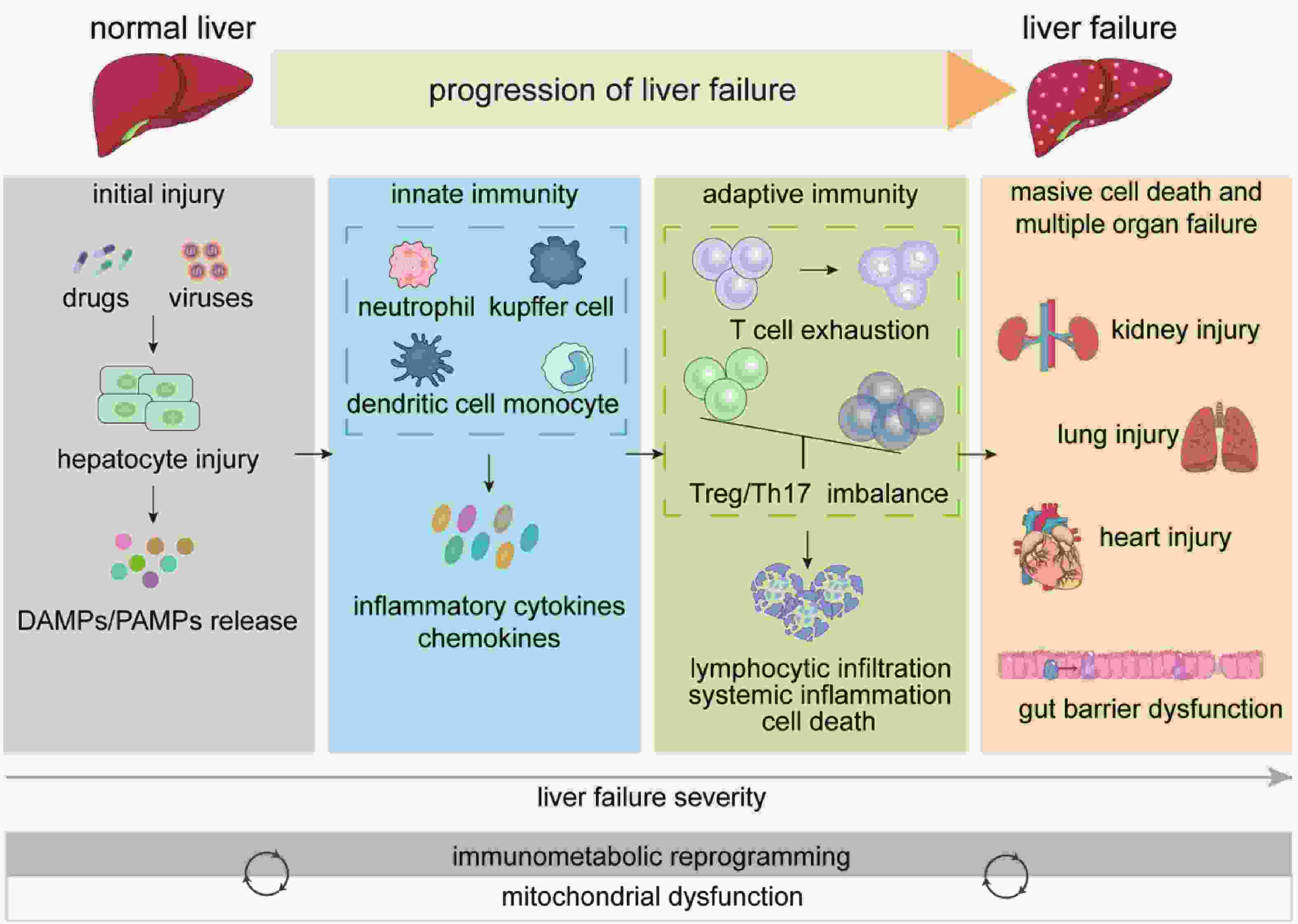
 下载:
下载: