Expression of Zinc Finger Transcription Factor Sall4 In Pancreatic Cancer and Its Impact on Cell Invasion and Migration
-
摘要:
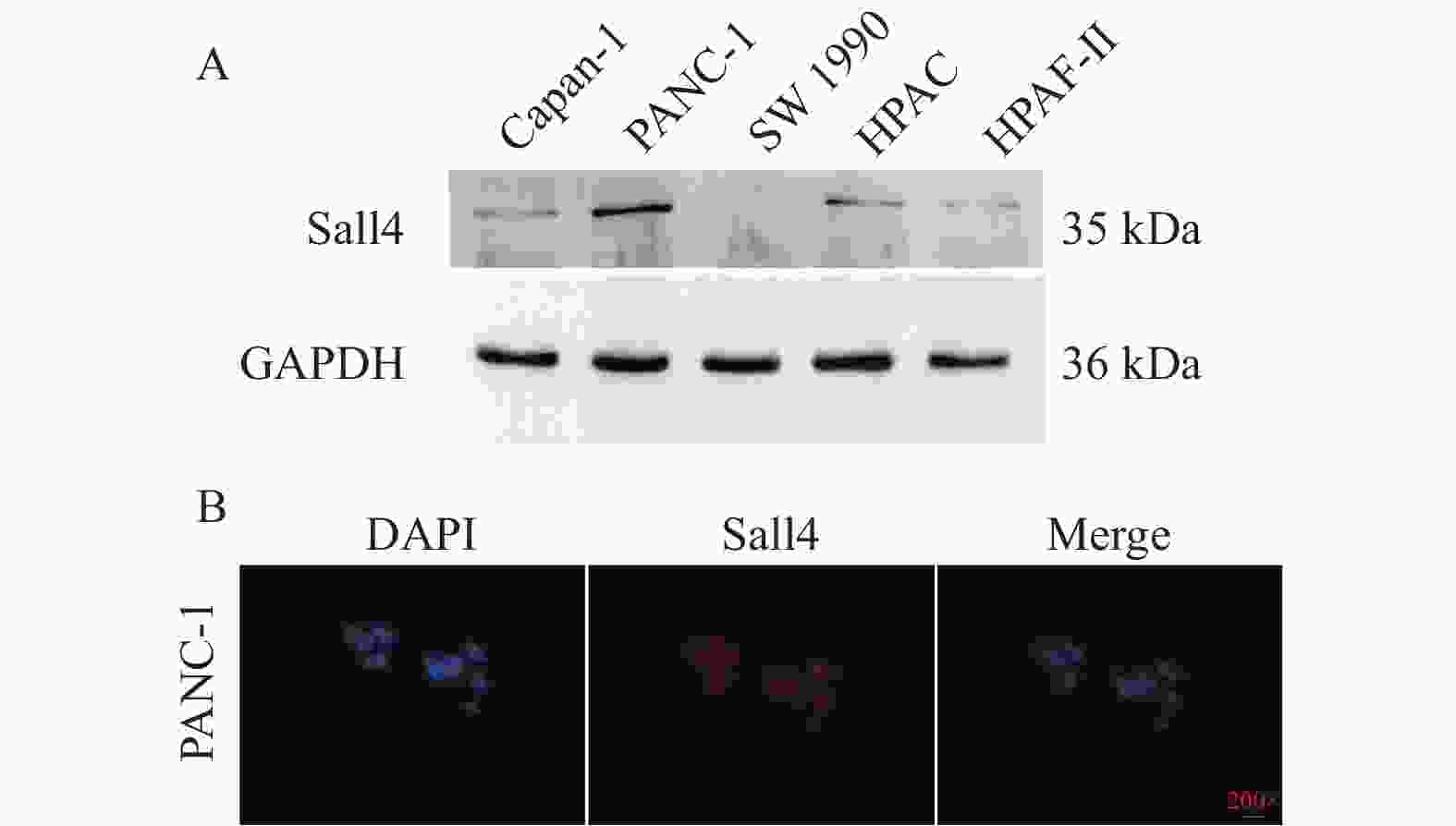
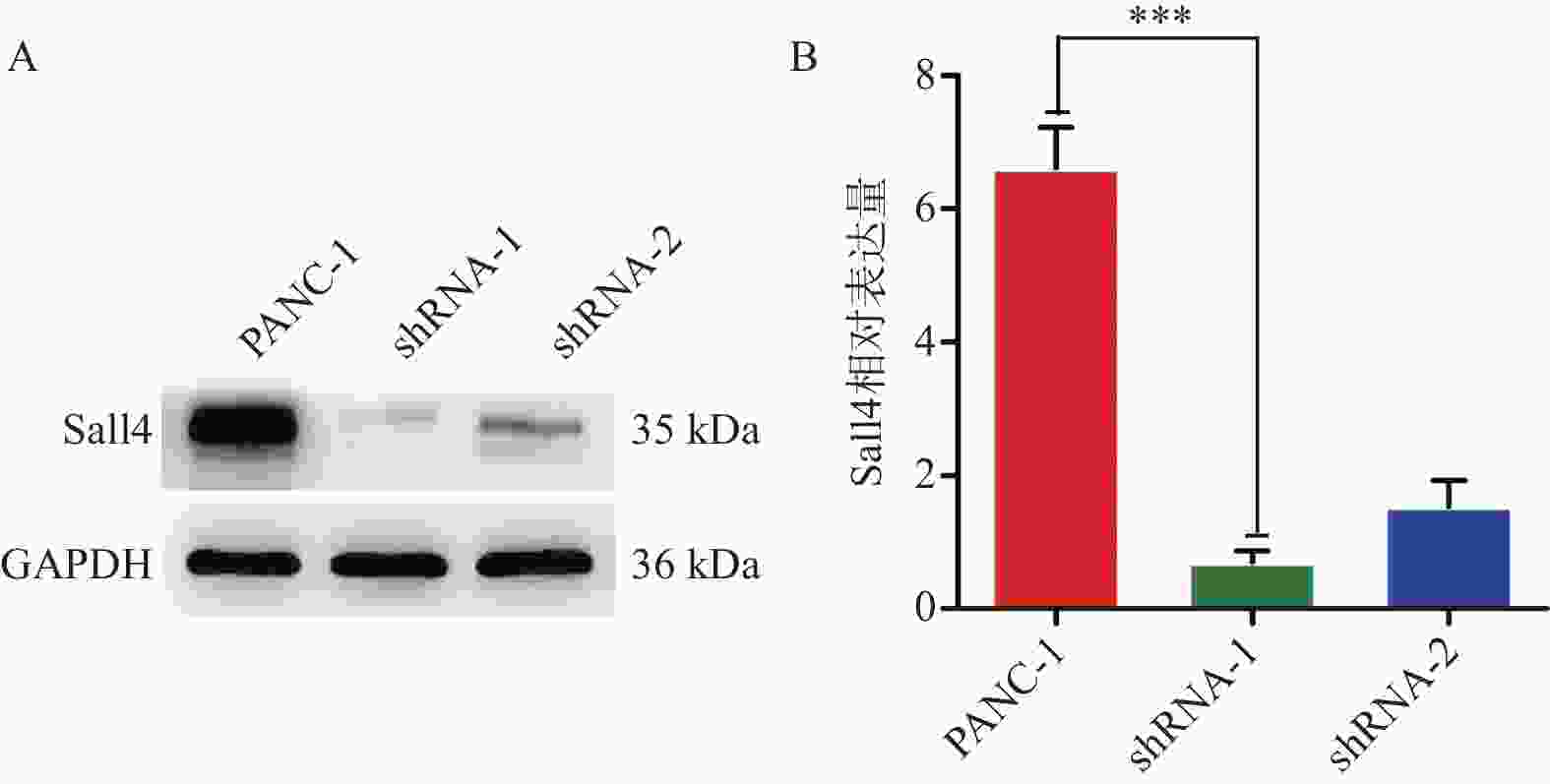
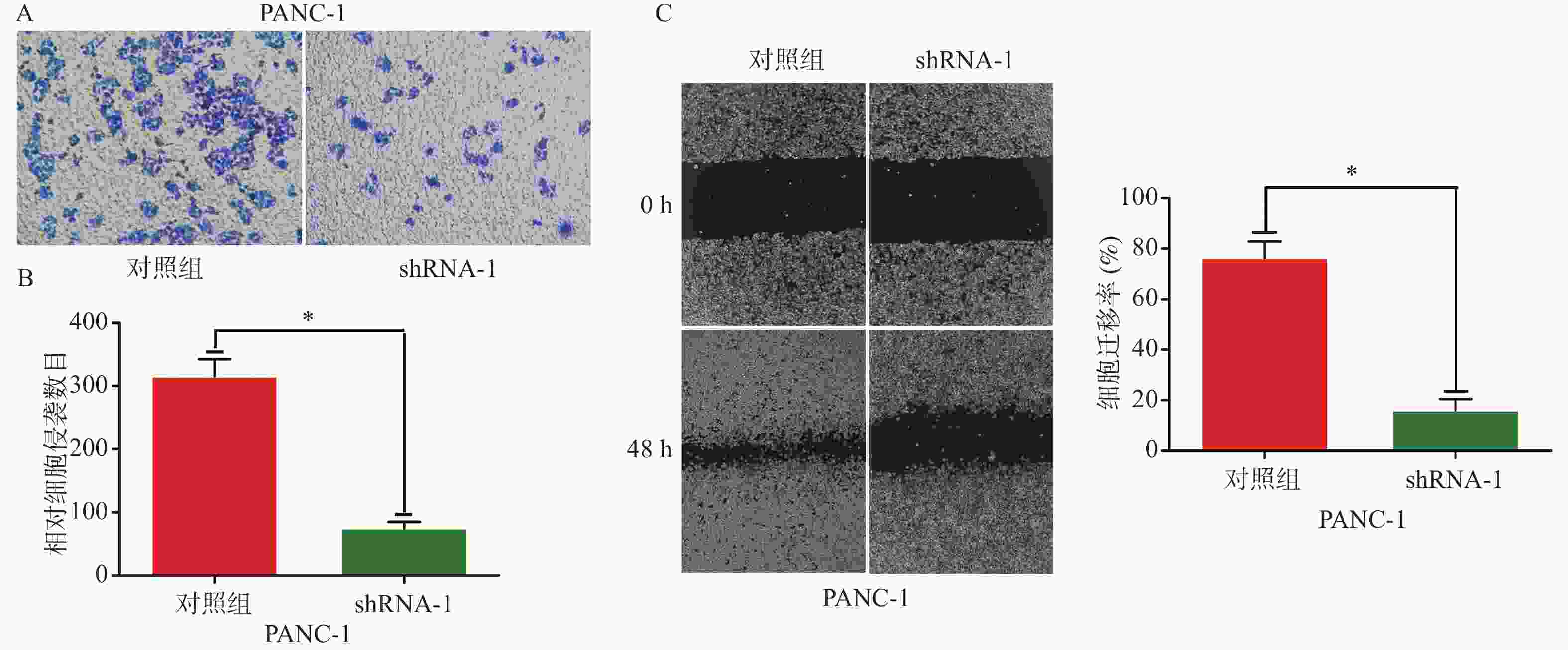
目的 研究胰腺癌组织中锌指转录因子4(spalt like transcription factor 4,Sall4)的表达,探究抑制其基因表达后对胰腺癌细胞侵袭和迁移的影响。 方法 选取2014年1月至2018年1月昆明医科大学第二附属医院诊治的64例胰腺癌患者的肿瘤组织及癌旁样本进行研究。通过免疫组织化学染色检测胰腺癌及癌旁组织中Sall4蛋白表达,并用实时定量PCR法检测癌和癌旁组织中Sall4 mRNA水平。同时,分析患者组织蜡块和临床资料与Sall4蛋白表达的关系。从胰腺癌细胞系中筛选高表达Sall4的细胞,分为对照组、shRNA-1组和shRNA-2组。用Sall4慢病毒(抑制基因序列)转染shRNA-1组和shRNA-2组,对照组仅转染试剂。通过Western blotting法检测Sall4表达量,并筛选出Sall4抑制明显的胰腺癌细胞进行侵袭性和迁移性能力研究; 结果 64例胰腺癌组织样本中有28例Sall4(43.8%)呈阳性,蛋白表达阳性率高于癌旁组织5例(7.8%),强阳性有16例,中等至弱阳性12例。样本中有11例胰腺癌患者发生了复发,差异具有统计学意义(P < 0.05),且其表达与淋巴结转移情况、肿瘤的分化程度以及肿瘤的分期具有相关性。研究表明相比于癌旁组织,Sall4在胰腺癌组织中的表达更为明显(P < 0.05)。此外,胰腺癌组织中Sall4表达水平的升高可能与患者术后复发率的增加密切相关。shRNA-1组和shRNA-2组成功抑制了Sall4的表达,而shRNA-1组的抑制效果更明显。与对照组相比,shRNA-1组的SW480细胞侵袭和迁移能力降低(P < 0.05)。 结论 胰腺癌患者的Sall4表达越高,术后越容易复发;抑制Sall4的表达能够显著抑制胰腺癌的细胞侵袭和迁移能力。 -
关键词:
- 胰腺癌 /
- 锌指转录因子Sall4 /
- 细胞侵袭 /
- 细胞迁移
Abstract:Objective To investigate the expression of spalt like transcription factor 4 (Sall4) in pancreatic cancer tissues and its clinical significance, as well as the impact of inhibiting its gene expression on the migration and invasion of pancreatic cancer cells. Methods This study involved 64 patients with pancreatic cancer treated at the 2nd Affiliated Hospital of Kunming Medical University from January 2014 to January 2018. Immunohistochemical staining was used to detect the expression of Sall4 protein in adjacent non-tumor tissues and pancreatic cancer tissues, respectively. Real-time quantitative PCR was used to measure the mRNA expression levels of Sall4 in the cancerous and adjacent non-tumor tissues, respectively. The relationship between the clinicopathological data of these patients and the expression of Sall4 protein was also analyzed. The cells of high Sall4 expression were screened from the human pancreatic cancer cell lines PANC-1, Capan-1, SW 1990, HPAC, and HPAF-II. The pancreatic cancer cells of high Sall4 expression were divided into three groups: control group, shRNA-1 group, and shRNA-2 group. The shRNA-1 and shRNA-2 groups were transfected with the corresponding Sall4 lentiviral inhibitory gene sequences, while the control group was transfected with the reagent without an interference sequence. Western blotting was used to measure the expression of Sall4, and cells with significantly reduced expression were selected for subsequent experiments. The transfected cells were then assessed for their invasive and migratory abilities. Results Among the 64 samples of pancreatic cancer tissues, 28 cases (43.8%) were positive for Sall4 expression, a rate significantly higher than 5 cases (7.8%) in adjacent non-tumor tissues. Furthermore, 16 cases exhibited strong positivity, while 12 cases showed moderate or weak positivity. Recurrence occurred in 11 pancreatic cancer patients. The difference was statistically significant. The expression of Sall4 was correlated with tumor differentiation, staging, and lymph node metastasis, suggesting that Sall4 positivity might be an independent risk factor affecting the prognosis of pancreatic cancer. Among the five cell lines, PANC-1 had the highest relative expression of Sall4 and was selected for further experiments. The shRNA-1 and shRNA-2 groups successfully suppressed the expression of Sall4, with the shRNA-1 group showing a more pronounced effect. Compared to the control group, the invasive and migratory abilities of SW480 cells were significantly reduced in the shRNA-1 group (P < 0.05). Conclusion Sall4 is highly expressed in pancreatic cancer tissues, and higher expression is associated with a greater likelihood of postoperative recurrence. Inhibiting the expression of Sall4 can significantly suppress the invasion and migration abilities of human pancreatic cancer cells. -
Key words:
- Pancreatic cancer /
- Zinc finger transcription factor 4 /
- Cell invasion /
- Cell migration
-
图 1 胰腺癌患者Sall4免疫组化表达及对应组织HE染色情况(×200)
A:胰腺癌组织HE染色; B:胰腺癌组织Sall4染色; C:胰腺癌癌旁组织HE染色染色; D:胰腺癌癌旁组织Sall4染色; 注:A~D组均为肿瘤未复发组;E:胰腺癌组织HE染色; F:胰腺癌组织Sall4染色; G:胰腺癌癌旁组织HE染色染色;H:胰腺癌癌旁组织Sall4染色; E-H组均为肿瘤复发组。
Figure 1. Immunohistochemical expression of Sall4 in pancreatic cancer patients and corresponding HE staining of tissues (×200)
表 1 Sall4蛋白表达与临床病理特征的关系[n(%)]
Table 1. Relationship between Sall4 protein expression and clinicopathological features[n(%)]
类别 n SALL4阳性 χ2值 P值 性别 男 48 17 1.29 0.256 女 16 11 年龄(岁) ≤ 60 29 18 2.10 0.148 > 60 35 10 肿瘤直径(cm) ≤ 4 42 10 5.93 0.015** > 4 22 18 肿瘤位置 胰头 46 26 3.883 0.049** 胰体尾 18 2 肿瘤分化程度 高中分化 45 12 5.119 0.024** 低分化 19 16 肿瘤 AJCC 分期 Ⅰ 30 23 8.529 0.004*** Ⅱ~Ⅲ 34 5 淋巴结转移 有 31 21 4.564 0.038** 无 33 7 ** P < 0.05; *** P < 0.005。 -
[1] 中华人民共和国国家卫生健康委员会医政医管局. 胰腺癌诊疗指南2022年版)[J]. 中华消化外科杂志,2022,21(9):1117-1136. doi: 10.3760/cma.j.cn115610-20220726-00431 [2] 王军华,王锐炫. 胰腺癌新辅助化疗现状和治疗策略. 中华肝脏外科手术学电子杂志,2024,13(5) : 640-643. [3] Fatma A. Abouelnazar,Xiaoxin Zhang,Maoye Wang,et al. The new advance of SALL4 in cancer: Function,regulation,and implication[J]. Journal of Clinical Laboratory Analysis,2023,37(9):1-16. [4] Zhang Y,Bharadwaj U,Logsdon C D ,et al. ZIP4 regulates pancreatic cancer cell growth by activating IL-6/STAT3 pathway through zinc finger transcription factor CREB. [J]. Clinical Cancer Research,2010,16(5): 1423-1430. [5] Maity G,Haque I,Ghosh A,et al. The MAZ transcription factor is a downstream target of the oncoprotein Cyr61/CCN1 and promotes pancreatic cancer cell invasion via CRAF-ERK signaling[J]. Journal of Biological Chemistry,2018,293(12):4334-4349. [6] Wei C Y,Zhu M X,Zhang P F,et al. PKCα/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma[J]. J Hepatol,2022,77(1):163-176. [7] Huang W,Li N,Hu J,et al. Inhibitory effect of RNA-mediated knockdown of zinc finger protein 91 pseudogene on pancreatic cancer cell growth and invasion[J]. Oncology Letters,2016,12(5):1343-1348. [8] Shidan Liu,Jiaxi Luan,Yan Ding,et al. miR-144-3p Targets FosB Proto-oncogene,AP-1Transcription Factor Subunit (FOSB) to Suppress Proliferation,Migration,and Invasion of PANC-1 Pancreatic Cancer Cells[J]. Oncology Research,2018,26(5):683-690. doi: 10.3727/096504017X14982585511252 [9] Inaguma S,Ito H,Riku M ,et al. Addiction of pancreatic cancer cells to zinc-finger transcription factor ZIC2[J]. Oncotargt,2015,6(29): 28257-28268; [10] Vienot A,Monnien F,Truntzer C,et al. SALL4-related gene signature defines a specific stromal subset of pancreatic ductal adenocarcinoma with poor prognostic features[J]. Mol Oncol,2023,17(7):1356-1378. [11] Jung J,Park S,Jang Y,et al. Clinical significance of glycolytic metabolic activity in hepatocellular carcinoma[J]. Cancers (Basel),2022,15(186):1-14. [12] Ding Y,Cho M,Chan A,et al. SALL4 is expressed in pancreatic acinar cell carcinoma[J]. Case Reports in Clinical Pathology,2014,1(2):36-41; [13] Hotz B,Visekruna A,Buhr H J,et al. Beyond epithelial to mesenchymal transition: a novel role for the transcription factor snail in inflammation and wound healing[J]. Journal of Gastrointestinal Surgery,2010,14(2):388-397. doi: 10.1007/s11605-009-1068-3 [14] Dengfeng C,Humphrey P A,Allan R W. SALL4 is a novel sensitive and specific marker for metastatic germ cell tumors,with particular utility in detection of metastatic yolk sac tumors[J]. Cancer,2019,12(115):2640-2651. [15] Akimasa Seno,Tomonari Kasai,Masashi Ikeda,et al. Characterization of Gene Expression Patterns among Artificially Developed Cancer Stem Cells Using Spherical Self-Organizing Map[J]. Cancer Informatics,2016,15(5):163-178. [16] Sherma Zibadi et al. “SALL4 expression in gastrointestinal and pancreatic adenocarcinoma and hepatocellular carcinoma: an immunohistochemical study”[J]. American Journal of Clinical Pathology,2014(1):214-218. [17] Xingding Zhang,Jing Yu,Yang Qin,et al. The zinc finger transcription factor ZKSCAN3 promotes prostate cancer cell migration[J]. The International Journal of Biochemistry & Cell Biology,2012,44(7):1166-1173. -






 下载:
下载: