Advances in the Study of Varenicline Combined with Nicotine Patches for the Treatment of Tobacco Dependence
-
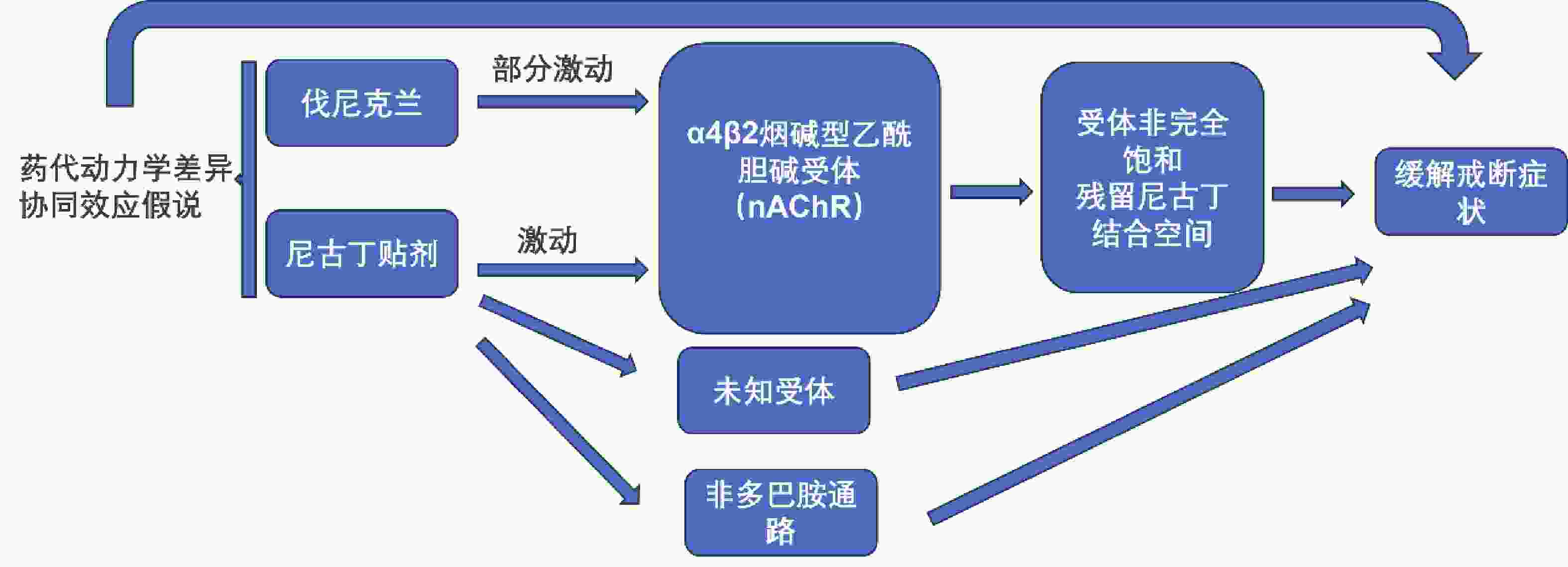
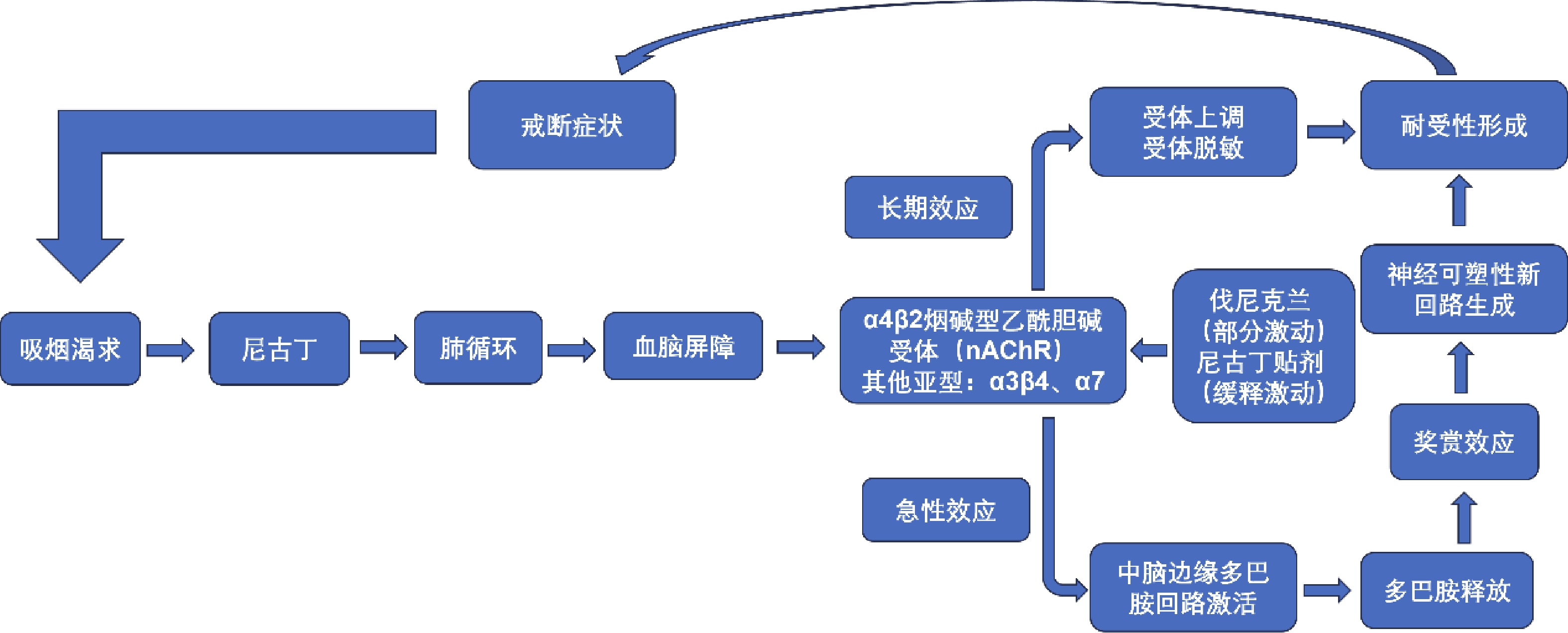
摘要: 中国吸烟人数居世界首位,每年因烟草死亡人数超过100万,戒烟是降低吸烟相关疾病患病率及疾病早死率的可行干预措施。现国内已批准使用的戒烟疗法对部分烟草依赖患者疗效欠佳,为进一步提高戒烟率,可选择联合用药,其中有伐尼克兰联合尼古丁替代疗法,但现有研究关于联合治疗的效果及安全性的评估结论存在矛盾,现就伐尼克兰联合尼古丁替代疗法治疗烟草依赖的作用机制、疗效及安全性进行综述,旨在为该联合治疗提供依据。Abstract: China has the highest number of smokers in the world with more than 1 million tobacco-related deaths each year. Smoking cessation is a feasible intervention to reduce the prevalence of smoking-related diseases and premature deaths. The smoking cessation therapies approved for use in China are ineffective for some tobacco dependent patients. In order to further improve the rate of smoking cessation, a combination of medications can be chosen, including varenicline combined with nicotine replacement therapy. However, there are conflicting evaluation conclusions on the effectiveness and safety of combination therapy. This article aims to review the mechanism of action, efficacy, and safety of varenicline combined with nicotine replacement therapy in the treatment of tobacco dependence, aiming to provide a basis for this combination therapy.
-
Key words:
- Varenicline /
- Nicotine patch /
- Tobacco dependence /
- Combination therapy /
- Efficacy
-
表 1 伐尼克兰联合尼古丁贴剂临床试验汇总
Table 1. Varenicline combined with nicotine patch clinical trial summary
临床试验/研究
信息样本量 FTND
评分a纳入人群 用药方案 主要结局指标 不良反应 Koegelenberg[12] 435 4.5 日吸烟量≥10支
的成人在TQDb前2周开始尼古丁贴片(15 mg/16 h),并持续12周(NRT总疗程14周)。伐尼克兰在TQD前1周开始使用:第1~3日0.5 mg qd,第4~7日0.5 mg bid,
第8日起1 mg bid持续使用12周,并在第13周时减停(伐尼克兰总疗程14周)。治疗第9~12周经呼出一氧化碳确认(≤10 ppm)的4周持续戒断率。 在联合治疗组中,恶心、睡眠障碍、皮肤反应、便秘和抑郁的发生率更高,但只有皮肤反应达到统计学意义(14.4% vs 7.8%, P = 0.03)。 King[17] 122 3.9 日吸烟量5~30支且酗酒(男性每周饮酒>14杯,女性每周饮酒>7杯,过去1年每月≥1个酗酒日 尼古丁贴片在TQD早晨开始使用,并按照生产商推荐的剂量持续使用 10 周。轻度吸烟者(每日吸烟量<10支):前6 周每天使用 14 mg的贴片,之后 4 周每天使用 7 mg的贴片。重度吸烟者(每日吸烟量≥10 支):前6 周每天使用 21 mg的贴片,之后 2 周每天使用14 mg的贴片,再之后 2 周每天使用7 mg的贴片。伐尼克兰在TQD开始使用:第1~3日0.5 mg qd,第4~7日0.5 mg bid,第8日起1 mg bid持续使用12周,在第13周时减停。 自我报告的治疗第9~12 周持续戒断率(定义为:完全不吸一口烟);第12周时经呼出一氧化碳确认(≤10 ppm)戒烟。 与单药治疗相比,联合治疗组的参与者出现恶、胀气、梦境异、睡眠问题和头痛的发生率较高。 Hajek[11] 117 4.9 成人吸烟者 在TQD当日开始尼古丁贴片(15 mg/16 h),并持续4周。伐尼克兰在TQD前1周开始使用:第1~3日0.5 mg qd,第4~7日0.5 mg bid,第8日起1 mg bid持续使用至第12周(伐尼克兰总疗程13周)。 治疗第1~4周经呼出一氧化碳确认(<9 ppm)的4周持续戒断率。 在联合治疗组中,梦境异常的发生率相对较高,但未达统计学意义。 Ramon[13] 341 6.5 成人且近6个月
日吸烟量≥20支在TQD当日开始尼古丁贴片(21 mg/24h),并持续12周。伐尼克兰在TQD前1周开始使用:第1~3日0.5 mg qd,第4~7日0.5 mg bid,第8日起1 mg bid持续使用至第12周。 治疗第2~12周经呼出一氧化碳确认(<10 ppm)持续戒断率。 两组失眠、梦境异常、恶心为最常见不良反应,发生率差异无统计学意义。联合治疗组头痛发生率相对较高,但两组差异无统计学意义。 Baker[14] 1251 5.0 成人且过去6个月
日吸烟量≥5支;呼出一氧化碳≥5 ppm在TQD前2周开始尼古丁贴片(14 mg/24 h),并持续12或24周(NRT总疗程14或26周)。伐尼克兰在TQD前1周开始使用:第1~3日0.5 mg qd,第4~7日0.5 mg bid,第8日起1 mg bid持续使用11或23周(伐尼克兰总疗程12或24周)。 TQD后第52周经呼气一氧化碳验证(≤
5 ppm)的7 d时点
戒烟率。在4个治疗组中,最常见的不良事件是恶心、失眠和情绪变化。 aFTND评分:Fagerstrom尼古丁依赖评估量表评分,0~3分轻度,4~6分中度,7~10分重度;bTQD: Target Quit Day,目标戒烟日。 -
[1] 王辰,肖丹,池慧. 中国吸烟危害健康报告2020概要[J]. 中国循环杂志,2021,36(10):937-952. [2] Siegel R L,Miller K D,Jemal A. Cancer statistics,2019[J]. CA Cancer J Clin,2019,69(1):7-34. doi: 10.3322/caac.21551 [3] Ebbert J O,Hays J T,Hurt R D. Combination pharmacotherapy for stopping smoking: What advantages does it offer?[J]. Drugs,2010,70(6):643-650. doi: 10.2165/11536100-000000000-00000 [4] Kangle G ,Liying Z ,Xue S ,et al. Varenicline and related interventions on smoking cessation: A systematic review and network meta-analysis [J]. Drug and Alcohol Dependence,2022,241(12): 109672. [5] 中华人民共和国国家卫生健康委员会. 中国临床戒烟指南(2015年版)[J]. 临床指南汇编数据库,2019: e52-e70. [6] Benowitz L N. Pharmacology of nicotine: Addiction,moking-induced disease,and therapeutics[J]. Annual Review of Pharmacology and Toxicology,2009,49(2):57-71. [7] Benowitz N L. Nicotine addiction[J]. N Engl J Med,2010,362(24):2295-2303. [8] Giulietti F,Filipponi A,Rosettani G,et al. Pharmacological approach to smoking cessation: An updated review for daily clinical practice[J]. High Blood Press Cardiovasc Prev,2020,27(5):349-362. doi: 10.1007/s40292-020-00396-9 [9] Jordan C J,Xi Z X. Discovery and development of varenicline for smoking cessation[J]. Expert Opin Drug Discov,2018,13(7):671-683. doi: 10.1080/17460441.2018.1458090 [10] Ebbert J O,Burke M V,Hays J T,et al. Combination treatment with varenicline and nicotine replacement therapy[J]. Nicotine Tob Res,2009,11(5):572-576. [11] Peter H ,Myers K S ,Al-Rehan D ,et al. Is a combination of varenicline and nicotine patch more effective in helping smokers quit than varenicline alone? A randomised controlled trial [J]. BMC Medicine,2013,11(1): 140-147 [12] Koegelenberg C F,Noor F,Bateman E D,et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: A randomized clinical trial[J]. JAMA,2014,312(2):155-161. doi: 10.1001/jama.2014.7195 [13] M J R ,Sergio M ,Antoni B ,et al. Combining varenicline and nicotine patches: A randomized controlled trial study in smoking cessation [J]. BMC Medicine,2014,12(1): 172-181. [14] Baker T B,Piper M E,Smith S S,et al. Effects of combined varenicline with nicotine patch and of extended treatment duration on smoking cessation: A randomized clinical trial[J]. JAMA,2021,326(15):1485-1493. [15] Lotfipour S,Mandelkern M,Alvarez-Estrada M,et al. A single administration of low-dose varenicline saturates α4β2* nicotinic acetylcholine receptors in the human brain[J]. Neuropsychopharmacology,2012,37(7):1738-1748. doi: 10.1038/npp.2012.20 [16] García-Gómez L, Hernández-Pérez A, Noé-Díaz V, et al. Smoking cessation treatmentis: Current psychological and pharmacological options [J]. Rev Invest Clin, 2019, 71(1): 7-16.García-Gómez L,Hernández-Pérez A,Noé-Díaz V,et al. Smoking cessation treatmentis: Current psychological and pharmacological options [J]. Rev Invest Clin,2019,71(1): 7-16. [17] King A,Vena A,De Wit H,et al. Effect of combination treatment with varenicline and nicotine patch on smoking cessation among smokers who drink heavily: A randomized clinical trial[J]. JAMA Netw Open,2022,5(3):e220951. [18] Chang P H ,Chiang C H ,Ho W C ,et al. Combination therapy of varenicline with nicotine replacement therapy is better than varenicline alone: A systematic review and meta-analysis of randomized controlled trials[J]. Bmc Public Health,2015,15(1): 689-697. [19] Leone F T,Zhang Y,Evers-Casey S,et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American thoracic society clinical practice guideline[J]. Am J Respir Crit Care Med,2020,202(2):e5-e31. [20] Shields P G,Bierut L,Arenberg D,et al. Smoking cessation,version 3.2022,NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw,2023,21(3):297-322. [21] Weeks R G ,Gobarani K R ,Abramson J M ,et al. Varenicline and nicotine replacement therapy for smokers admitted to hospitals: A randomized clinical trial [J]. JAMA Network Open,2024,7(6): e2418120. [22] Taylor L,Claire R,Campbell K,et al. Fetal safety of nicotine replacement therapy in pregnancy: Systematic review and meta-analysis[J]. Addiction,2021,116(2):239-277. [23] Coleman T. Recommendations for the use of pharmacological smoking cessation strategies in pregnant women[J]. CNS Drugs,2007,21(12):983-993. -





 下载:
下载: