Correlation between Serum IL-10,CTL and EB Virus Associated Infectious Mononucleosis Complicated with Liver Damage in Children
-
摘要:
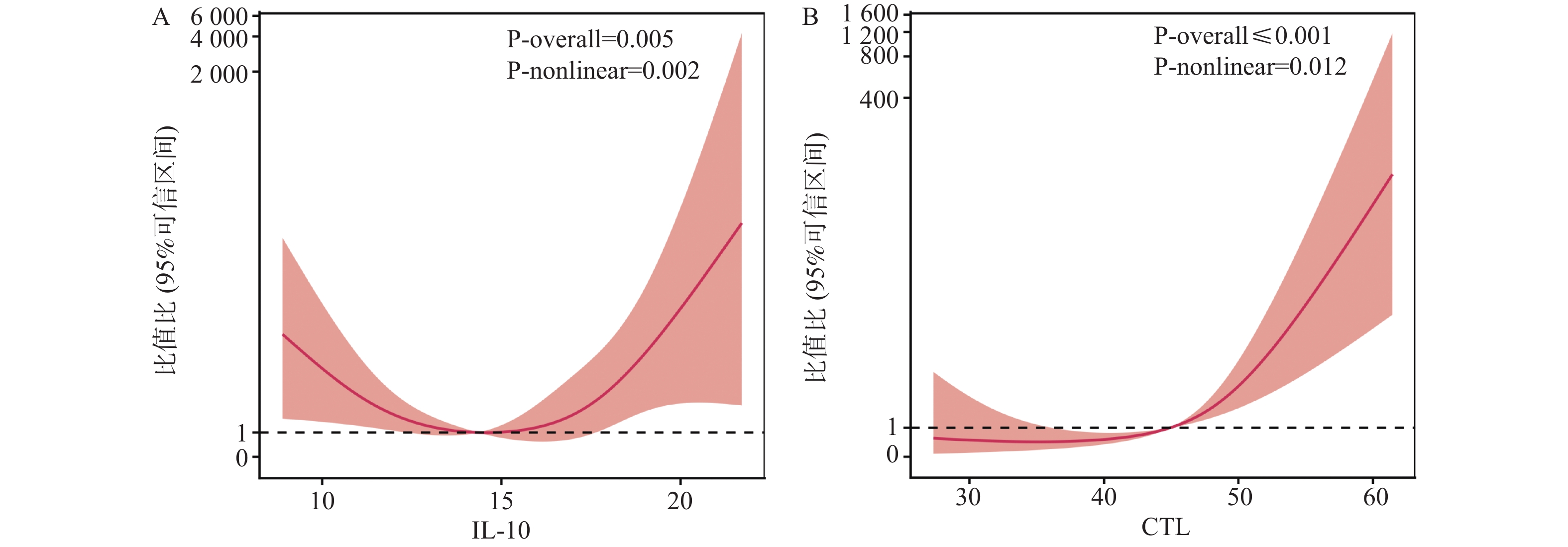
目的 研究血清白介素-10(IL-10)、细胞毒性T淋巴细胞(CTL)与儿童EB病毒(EBV)相关性传染性单核细胞增多症(IM)并发肝损害相关性。 方法 选取上海交通大学医学院附属上海儿童医学中心海南医院、苏州市吴江区儿童医院及河北省邯郸市第一医院2021年9月至2025年5月收治的215例EBV相关性IM患儿进行前瞻性研究,根据是否并发肝损害分为并发组(n = 86)、未并发组(n = 129)。另选同期215例健康儿童作为对照组。比较各组临床资料、血清IL-10、CTL水平,用限制性立方样条分析血清IL-10、CTL与儿童EB病毒相关性IM并发肝损害的相关性,Logistic回归分析儿童EB病毒相关性IM并发肝损害的相关影响因素,受试者工作特征曲线(ROC)分析血清IL-10、CTL及联合预测儿童EB病毒相关性IM并发肝损害的价值。 结果 EBV相关性IM患儿治疗前血清IL-10、CTL高于对照组(P < 0.05);并发组EBV DNA高于未并发组(P < 0.05);并发组治疗前和治疗3 d后IL-10、CTL显著高于未并发组,差异有统计学意义(P < 0.05);RCS模型分析发现,当节点数为3时,模型表现出最优拟合效果,似然比检验显著(P < 0.05),此时血清IL-10与儿童EB病毒相关性IM并发肝损害呈"U"形剂量-反应关系(P < 0.05),其高风险阈值14.90 pg/mL;CTL与儿童EB病毒相关性IM并发肝损害呈先平缓后上升的非线性关系(P < 0.05),其高风险阈值为46.10%;Logistic回归分析显示,校正了EBV DNA后,IL-10>14.90 pg/mL、CTL>46.10%仍是儿童EB病毒相关性IM并发肝损害的相关影响因素(P < 0.05);绘制ROC曲线显示,IL-10+CTL的AUC值(0.850,95%CI:0.795~0.895)显著高于两者单独的AUC值(P < 0.05);中度肝损害患儿治疗前和治疗3 d后IL-10、CTL显著高于轻度,差异有统计学意义(P < 0.05)。 结论 血清IL-10、CTL与EB病毒相关性IM并发肝损害相关,两者高风险阈值分别为14.90 pg/mL、46.10%,联合检测两者能提高对并发肝损害的预测价值,为临床提供指导。 -
关键词:
- 白介素-10 /
- 细胞毒性T淋巴细胞 /
- 儿童EB病毒相关性传染性单核细胞增多症 /
- 肝损害 /
- 相关性
Abstract:Objective To investigate the correlation between serum interleukin-10 (IL-10), cytotoxic T lymphocytes (CTL) and Epstein Barr virus (EBV)-associated infectious mononucleosis (IM) complicated with liver damage in children. Methods A prospective study was conducted on 215 children with EBV-associated IM admitted to Hainan Hospital of Shanghai Children's Medical Center, Shanghai Jiao Tong University School of Medicine, Children's Hospital of Wujiang District, Suzhou and Handan First Hospital from September 2021 to May 2025. Participants were divided into complicated group (with hepatic impairment) and non-complicated group (without hepatic impairment) based on the presence or absence of concurrent hepatic impairment. Additionally, 215 healthy children during the same period were selected as the control group. Clinical data, serum IL-10 and CTL levels were compared among groups. Restricted cubic spline (RCS) analysis was used to assess the correlation between serum IL-10, CTL, and hepatic impairment in children with EBV-associated IM. Logistic regression analysis was performed to identify relevant factors affecting hepatic impairment in children with EBV-associated IM. Receiver operating characteristic (ROC) curves were used to evaluate the predictive value of serum IL-10, CTL, and their combination for hepatic impairment in children with EBV-associated IM. Results Pre-treatment serum IL-10 and CTL levels in children with EBV-associated IM were higher than those in the control group (P < 0.05). EBV DNA in the complicated group was higher than in the non-complicated group (P < 0.05). Pre-treatment and 3-day post-treatment IL-10 and CTL levels in the complicated group were significantly higher than in the non-complicated group, with statistical significance (P < 0.05). RCS model analysis revealed optimal fitting when the number of knots was 3, with significant likelihood ratio test (P < 0.05). Serum IL-10 demonstrated a "U"-shaped dose-response relationship with hepatic impairment in children with EBV-associated IM (P < 0.05), with a high-risk threshold of 14.90 pg/mL. CTL showed a non-linear relationship with hepatic impairment, characterized by gradual stability followed by elevation (P < 0.05), with a high-risk threshold of 46.10%. Logistic regression analysis showed that after adjusting for EBV DNA, IL-10>14.90 pg/mL and CTL>46.10% remained independent risk factors for hepatic impairment in children with EBV-associated IM (P < 0.05). ROC curve analysis showed that the AUC value of IL-10+CTL (0.850, 95%CI: 0.795-0.895) was significantly higher than the individual AUC values of either marker (P < 0.05). Pre-treatment and 3-day post-treatment IL-10 and CTL levels in children with moderate hepatic impairment were significantly higher than in those with mild impairment, with statistical significance (P < 0.05). Conclusion Serum IL-10 and CTL are correlated with hepatic impairment in children with EBV-associated IM, with high-risk thresholds of 14.90 pg/mL and 46.10%, respectively. Combined detection of both markers enhances the predictive value for concurrent hepatic impairment and provides clinical guidance. -
表 1 表面标志染色抗体组合
Table 1. Surface marker staining antibody combination
标志物 荧光染料 功能 CD3 APC/Cyanine7 T细胞标记 CD8 PerCP/Cyanine5.5 CTL亚群标记 CD69 PE 早期活化标志 Live/Dead Zombie NIR 排除死细胞 表 2 EBV相关性IM患儿与对照组血清IL-10、CTL比较($ \bar x \pm s $)
Table 2. Comparison of serum IL-10 and CTL between EBV-associated IM children and control group ($ \bar x \pm s $)
组别 n IL-10(pg/mL) CTL(%) EBV相关性IM 215 15.45 ± 5.07 46.05 ± 9.23 对照组 215 4.22 ± 0.98 10.17 ± 2.64 t 31.888 54.802 P <0.001* <0.001* *P < 0.001。 表 3 并发组与未并发组临床资料比较[($ \bar x \pm s $)/n(%)]
Table 3. Comparison of clinical data between two groups[ ($ \bar x \pm s $)/n (%)]
资料信息 并发组(n=86) 未并发组(n=129) t/χ2 P 年龄(岁) 5.12 ± 1.68 5.53 ± 1.80 −1.680 0.094 性别 0.256 0.613 男 47(54.65) 75(58.14) 女 39(45.35) 54(41.86) 体质量(kg) 20.56 ± 3.89 20.83 ± 4.16 −0.478 0.633 发病季节 1.077 0.783 春季 23(26.74) 36(27.91) 夏季 22(25.58) 33(25.58) 秋季 30(34.88) 49(37.98) 冬季 11(12.79) 11(8.53) 症状体征 发热 82(95.35) 126(97.67) 0.301 0.583 眼睑水肿 64(74.42) 92(71.32) 0.249 0.618 淋巴结肿大 71(82.56) 102(79.07) 0.399 0.527 咽颊炎 84(97.67) 121(93.80) 0.983 0.321 皮疹 5(5.81) 4(3.10) 0.391 0.532 脾大 61(70.93) 96(74.42) 0.319 0.572 肝大 13(15.12) 14(10.85) 0.854 0.355 抗EBV-CA-IgM阳性 83(96.51) 125(96.90) 0.055 0.814 抗EBV-CA-IgG阳性 86(100.00) 129(100.00) 0.000 1.000 反应性淋巴细胞 14.94 ± 1.60 14.82 ± 1.53 0.553 0.581 EBV DNA[lg拷贝/mL] 3.35 ± 0.37 3.01 ± 0.42 6.094 <0.001* *P < 0.001。 表 4 并发组与未并发组血清IL-10、CTL比较($ \bar x \pm s $)
Table 4. Comparison of serum IL-10 and CTL between two groups ($ \bar x \pm s $)
组别 n IL-10(pg/mL) CTL(%) 治疗前 治疗3 d后 出院时 治疗前 治疗3 d后 出院时 并发组 86 17.33 ± 4.16 22.83 ± 6.29a 9.13 ± 2.44ab 50.74 ± 7.35 62.28 ± 12.76b 26.70 ± 7.58ab 未并发组 129 14.20 ± 3.59 13.95 ± 4.71 8.70 ± 2.15ab 42.93 ± 9.78 41.23 ± 10.05 25.83 ± 6.34ab t 5.874 11.821 1.361 6.310 13.488 0.911 P <0.001* <0.001* 0.175 <0.001* <0.001* 0.363 与治疗前比较,aP < 0.05;与治疗3 d后比较,bP < 0.05;*P < 0.001。 表 5 RCS模型统计检验验证结果
Table 5. Verification results of RCS model statistical test
节点数量 AIC BIC 似然比检验 LRχ2 P 2 702.59 704.37 3.779 0.041* 3 681.34 685.22 4.856 0.029* 4 685.27 689.15 3.812 0.035* *P < 0.05。 表 6 Logistic回归分析赋值表
Table 6. Assignment table of Logistic regression analysis
影响因素 赋值 模型1 IL-10(pg/mL) ≤14.90=1,>14.90=2 CTL(%) ≤46.10=1,>46.10=2 模型2 IL-10(pg/mL) ≤14.90=1,>14.90=2 CTL(%) ≤46.10=1,>46.10=2 表 7 儿童EB病毒相关性IM并发肝损害Logistic回归分析
Table 7. Logistic regression analysis of EB virus associated IM complicated with liver damage in children
影响因素 β SE Wald χ2 OR 95%CI P 下限 上限 模型1 IL-10 1.191 0.415 8.236 3.290 2.668 4.058 <0.001* CTL 1.301 0.362 12.909 3.672 3.155 4.273 <0.001* 常数项 −6.120 0.095 17.543 — — — <0.001* 模型2 IL-10 1.165 0.384 9.207 3.207 2.625 3.917 CTL 1.282 0.329 15.176 3.603 3.117 4.164 常数项 −6.077 0.083 16.395 — — — <0.001* 模型1:未校正EBV DNA;模型2:校正了EBV DNA;*P < 0.001。 表 8 血清IL-10、CTL及联合预测儿童EB病毒相关性IM并发肝损害的价值
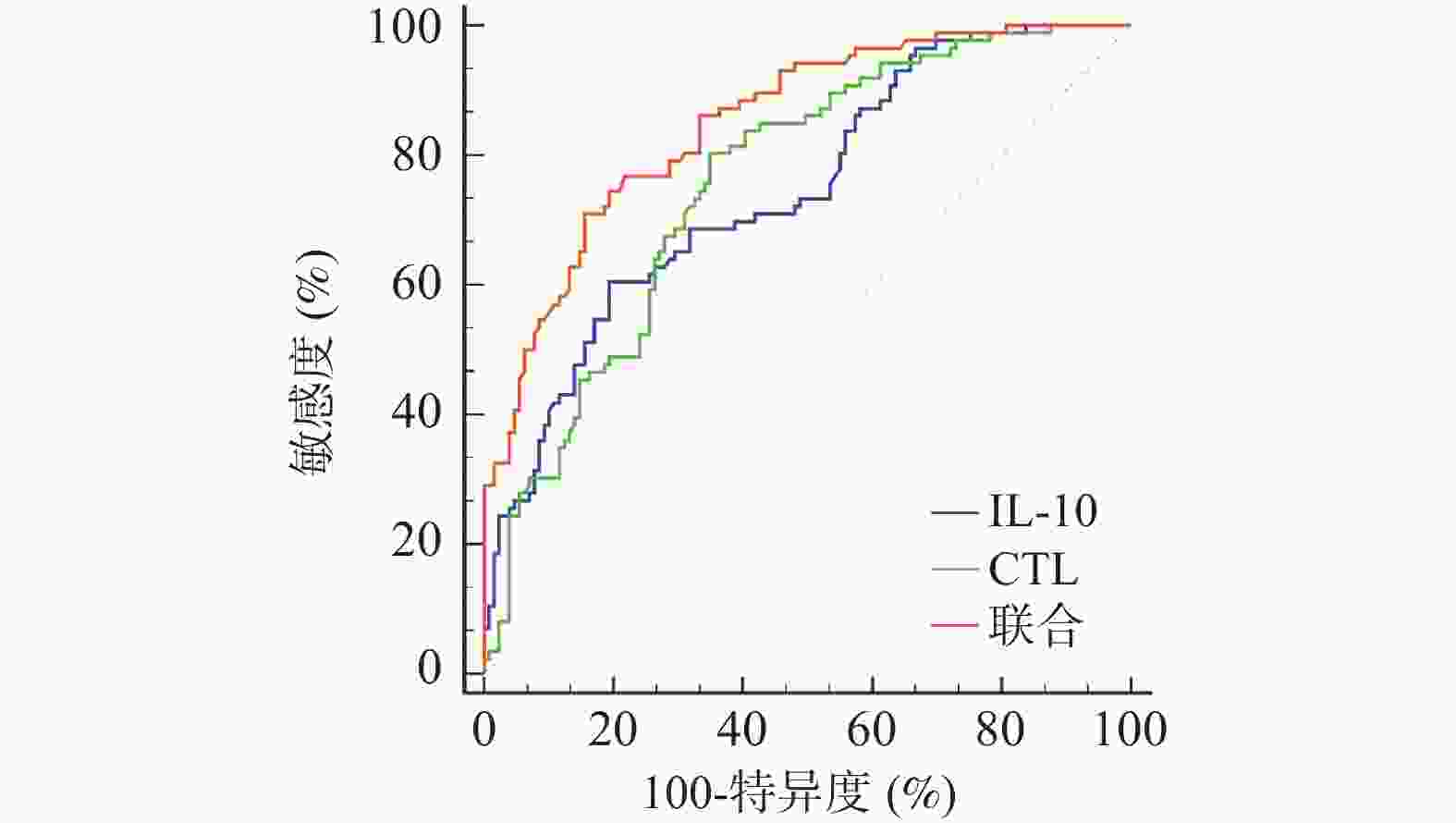
Table 8. The value of serum IL-10,CTL and combined prediction of EB virus-associated IM complicated with liver damage in children
指标 AUC 95%CI cut-off值 敏感度(%) 特异度(%) P IL-10 0.744 0.681~0.801 17.08 60.47 80.62 <0.001* CTL 0.758 0.695~0.814 46.13 80.23 65.12 <0.001* 联合 0.850 0.795~0.895 — 70.93 84.50 <0.001* *P < 0.001。 表 9 并发组不同肝损害程度患儿血清IL-10、CTL比较($ \bar x \pm s $)
Table 9. Comparison of serum IL-10 and CTL in children with different degrees of liver damage in the concurrent group ($ \bar x \pm s $)
肝损害程度 n IL-10(pg/mL) CTL(%) 治疗前 治疗3 d后 出院时 治疗前 治疗3 d后 出院时 中度 17 21.86 ± 3.95 29.47 ± 5.61a 9.35 ± 2.50ab 58.41 ± 6.02 73.85 ± 9.34b 27.88 ± 8.12ab 轻度 69 16.28 ± 3.50 21.06 ± 5.40a 9.07 ± 2.242ab 48.98 ± 6.50 59.65 ± 11.20b 26.38 ± 7.45ab t 5.740 5.709 0.451 5.432 4.824 0.731 P <0.001* <0.001* 0.653 <0.001* <0.001* 0.467 *P < 0.001;与治疗前比较,aP < 0.05;与治疗3 d后比较,bP < 0.05。 -
[1] Borghol A H, Bitar E R, Hanna A, et al. The role of Epstein-Barr virus in autoimmune and autoinflammatory diseases[J]. Crit Rev Microbiol, 2025, 51(2): 296-316. doi: 10.1080/1040841X.2024.2344114 [2] Li Y, Tang J, Ma Y, et al. Clinical significance and pathogenesis of GBP5 in infectious mononucleosis associated liver injury[J]. Ital J Pediatr, 2025, 51(1): 72. doi: 10.1186/s13052-025-01907-x [3] Liu Y, Wu Y, Liu D, et al. Infectious mononucleosis disguised as acute tonsillitis: Pay attention to liver function[J]. Asian J Surg, 2024, S1015-S9584(24): 01481-01487. [4] Zhang Y, Xie J, Zheng Y, et al. Infectious mononucleosis complicated by transitory Epstein-Barr virus infection of T and natural killer cells[J]. J Hematop, 2024, 17(3): 129-137. doi: 10.1007/s12308-024-00595-6 [5] 邵辉, 李杰, 马树民, 等. 甲状腺癌EB病毒感染与XRCC1、STING1、IL-10基因多态性及病理类型的关联[J]. 中华医院感染学杂志, 2024, 34(14): 2158-2162. [6] 靳丹丹, 周卫芳. 儿童EBV传染性单核细胞增多症细胞因子IL-6、γ-IFN、IL-10的改变[J]. 右江医学, 2024, 52(7): 607-612. [7] Fang C H, Cheng Y F, Lin S R, et al. Establishment of a protocol for rapidly expanding Epstein-Barr-virus-specific cytotoxic T cells with enhanced cytotoxicity[J]. BMC Cancer, 2024, 24(1): 980. doi: 10.1186/s12885-024-12707-7 [8] Aslan N, Watkin L B, Gil A, et al. Severity of acute infectious mononucleosis correlates with cross-reactive influenza CD8 T-cell receptor repertoires[J]. mBio, 2017, 8(6): e01841-e01847. [9] 中华医学会儿科学分会感染学组, 全国儿童EB病毒感染协作组. 儿童主要非肿瘤性EB病毒感染相关疾病的诊断和治疗原则建议[J]. 中华儿科杂志, 2016, 54(8): 563-568. [10] 中华医学会肝病学分会, 中华医学会消化病学分会. 常用肝脏生物化学试验的临床意义及评价共识[J]. 中华消化杂志, 2010, 18(5): 387-393. [11] Zhan Y, Fu Y, Dai H, et al. Characteristics and clinical significance of gut microbiota in patients with Epstein-Barr virus-associated liver dysfunction[J]. Microbiol Immunol, 2025, 69(4): 203-211. doi: 10.1111/1348-0421.13200 [12] 岳贇, 马丽, 王卫国. 学龄前儿童传染性单核细胞增多症合并肝功能异常的临床特征及影响因素[J]. 肝脏, 2024, 29(7): 852-856. doi: 10.14000/j.cnki.issn.1008-1704.2024.07.030 [13] Moyano A, Ferressini Gerpe N M, De Matteo E, et al. M1 macrophage polarization prevails in Epstein-Barr virus-infected children in an immunoregulatory environment[J]. J Virol, 2022, 96(1): e01434-21. [14] 周胜, 马晓慧, 孟晓飞. 血清白细胞介素10、腺苷脱氨酶与传染性单核细胞增多症患儿心肌损害的关系分析[J]. 中国卫生检验杂志, 2023, 33(14): 1718-1721. [15] 刘瑞坪, 陈彦妤, 王俊颖, 等. EB病毒vIL-10基因的表达与功能研究[J]. 中国病原生物学杂志, 2025, 20(2): 131-135, 140. doi: 10.13350/j.cjpb.250201 [16] Zhang Y, Huang C, Zhang H, et al. Characteristics of immunological events in Epstein-Barr virus infection in children with infectious mononucleosis[J]. Front Pediatr, 2023, 11: 1060053. doi: 10.3389/fped.2023.1060053 [17] Cai L, Xing Y, Xia Y, et al. Comparative study of biomarkers for the early identification of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in infectious mononucleosis[J]. BMC Infect Dis, 2023, 23(1): 728. doi: 10.1186/s12879-023-08654-6 [18] Zhu J, Cao N, Wu F, et al. Predicting 30-day mortality in hemophagocytic lymphohistiocytosis: Clinical features, biochemical parameters, and machine learning insights[J]. Ann Hematol, 2025, 104(4): 2239-2264. doi: 10.1007/s00277-025-06249-6 [19] Weng Y, Wang L, Wang Y, et al. Spatial organization of macrophages in CTL-rich hepatocellular carcinoma influences CTL antitumor activity[J]. Cancer Immunol Res, 2025, 13(3): 310-322. doi: 10.1158/2326-6066.CIR-24-0589 [20] Zhou Y, Wang D, Zhou L, et al. Cell softness renders cytotoxic T lymphocytes and T leukemic cells resistant to perforin-mediated killing[J]. Nat Commun, 2024, 15(1): 1405. doi: 10.1038/s41467-024-45750-w [21] Chiang S C C, Covill L E, Tesi B, et al. Efficacy of T-cell assays for the diagnosis of primary defects in cytotoxic lymphocyte exocytosis[J]. Blood, 2024, 144(8): 873-887. doi: 10.1182/blood.2024024499 [22] 秦爱华, 白桦, 孙秀丽, 等. IM患儿EBV DNA载量与外周血CTL细胞、TLRs表达和临床转归的关系[J]. 中华医院感染学杂志, 2023, 33(15): 2372-2376. [23] Liu L, Yang X, Li M, et al. EBV-DNA load: A predictor of immune and hepatic dysfunction in pediatric infectious mononucleosis[J]. Diagn Microbiol Infect Dis, 2025, 113(1): 116897. doi: 10.1016/j.diagmicrobio.2025.116897 [24] Gutiérrez-Guerrero A, Espinosa-Padilla SE, Lugo-Reyes SO. Anything that can go wrong: cytotoxic cells and their control of Epstein-Barr virus[J]. Rev Alerg Mex, 2024, 71(1): 29-39. doi: 10.29262/ram.v71i1.1276 [25] Latour S. Human immune responses to Epstein-Barr virus highlighted by immunodeficiencies[J]. Annu Rev Immunol, 2025, 43(1): 723-749. doi: 10.1146/annurev-immunol-082323-035455 [26] Rex D A B, Keshava Prasad T S, Kandasamy R K. Revisiting regulated cell death responses in viral infections[J]. Int J Mol Sci, 2022, 23(13): 7023. doi: 10.3390/ijms23137023 -





 下载:
下载: