Therapeutic Effects and Mechanisms of Emodin on Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice
-
摘要:
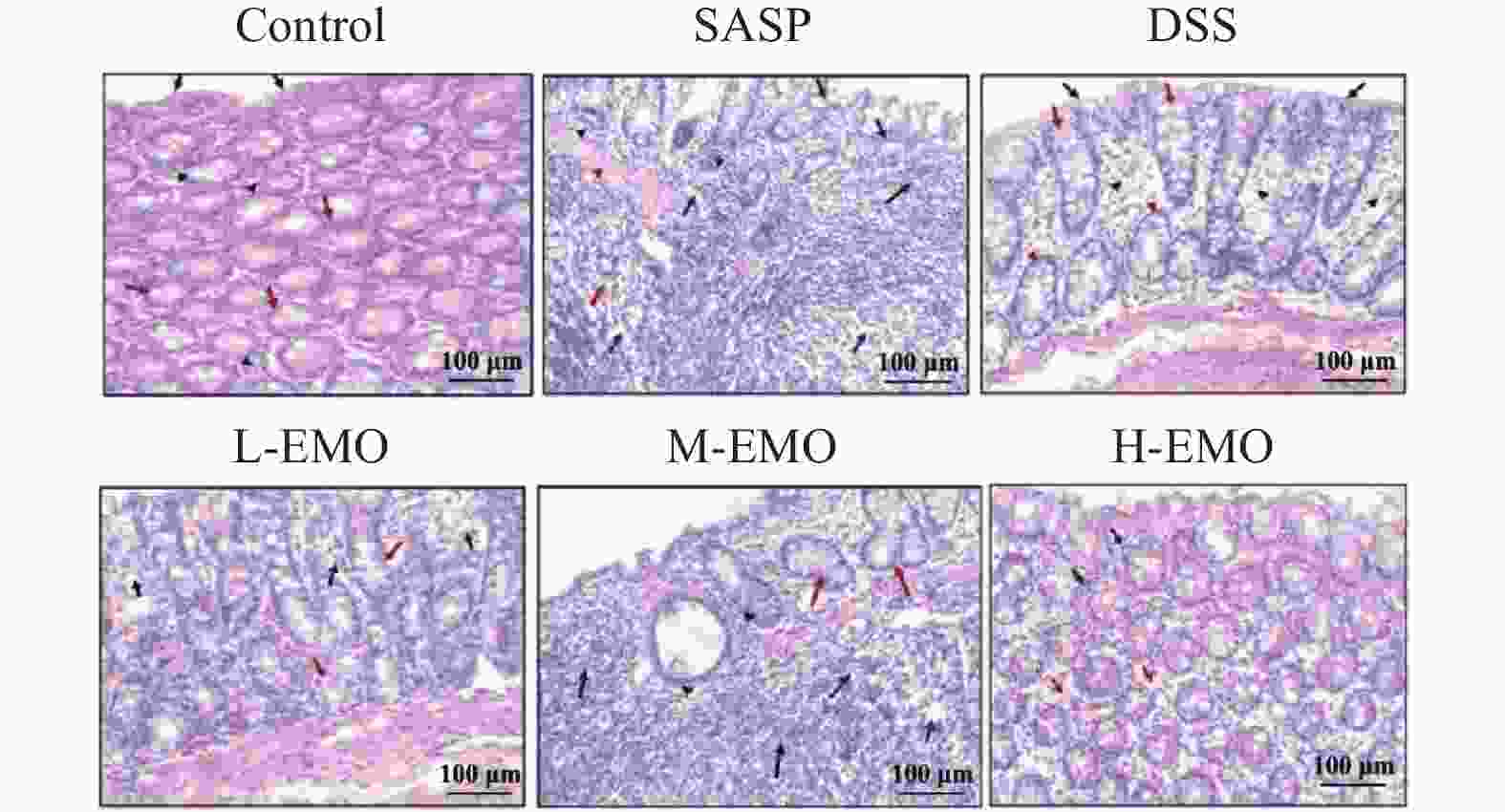
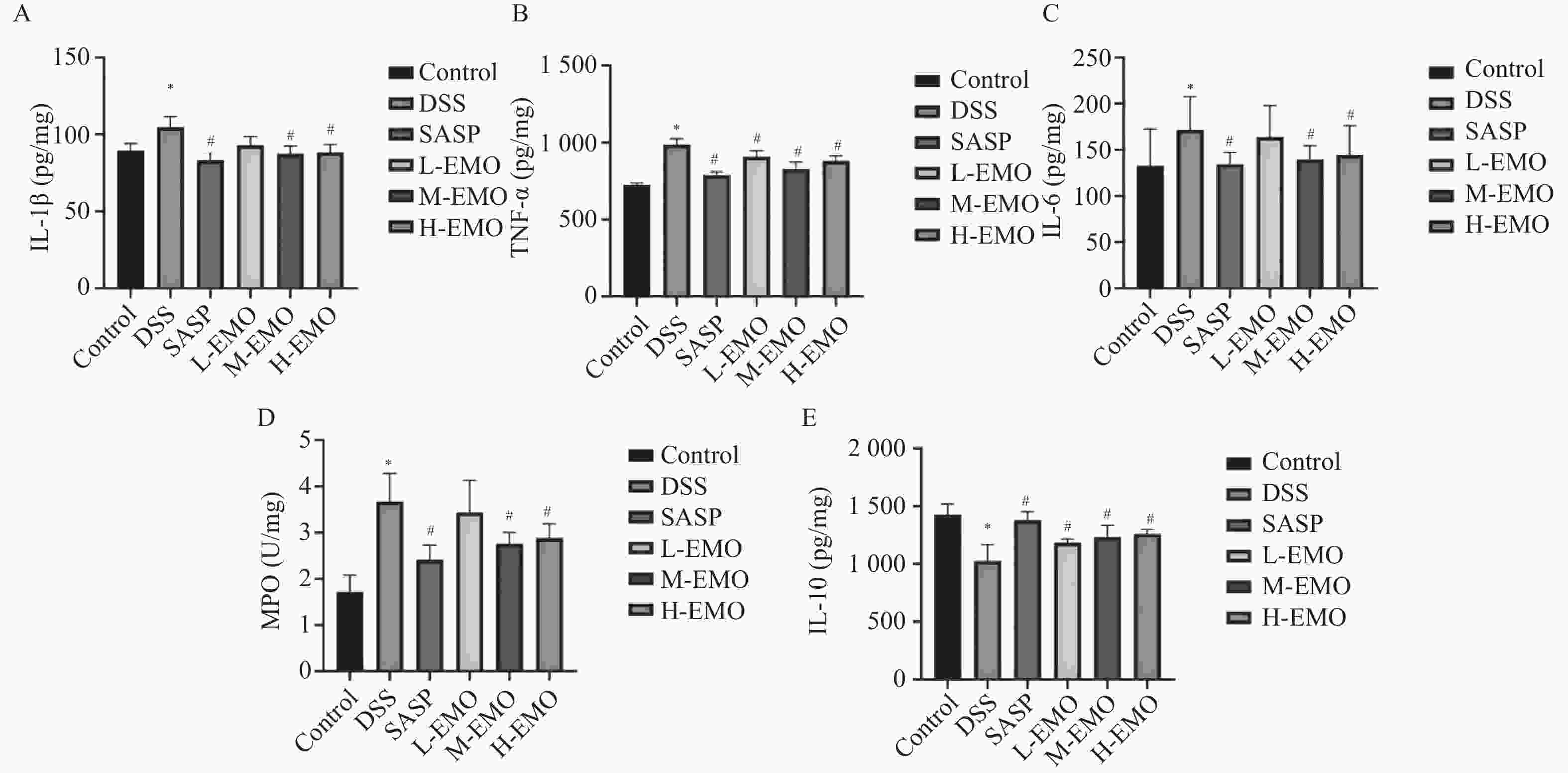
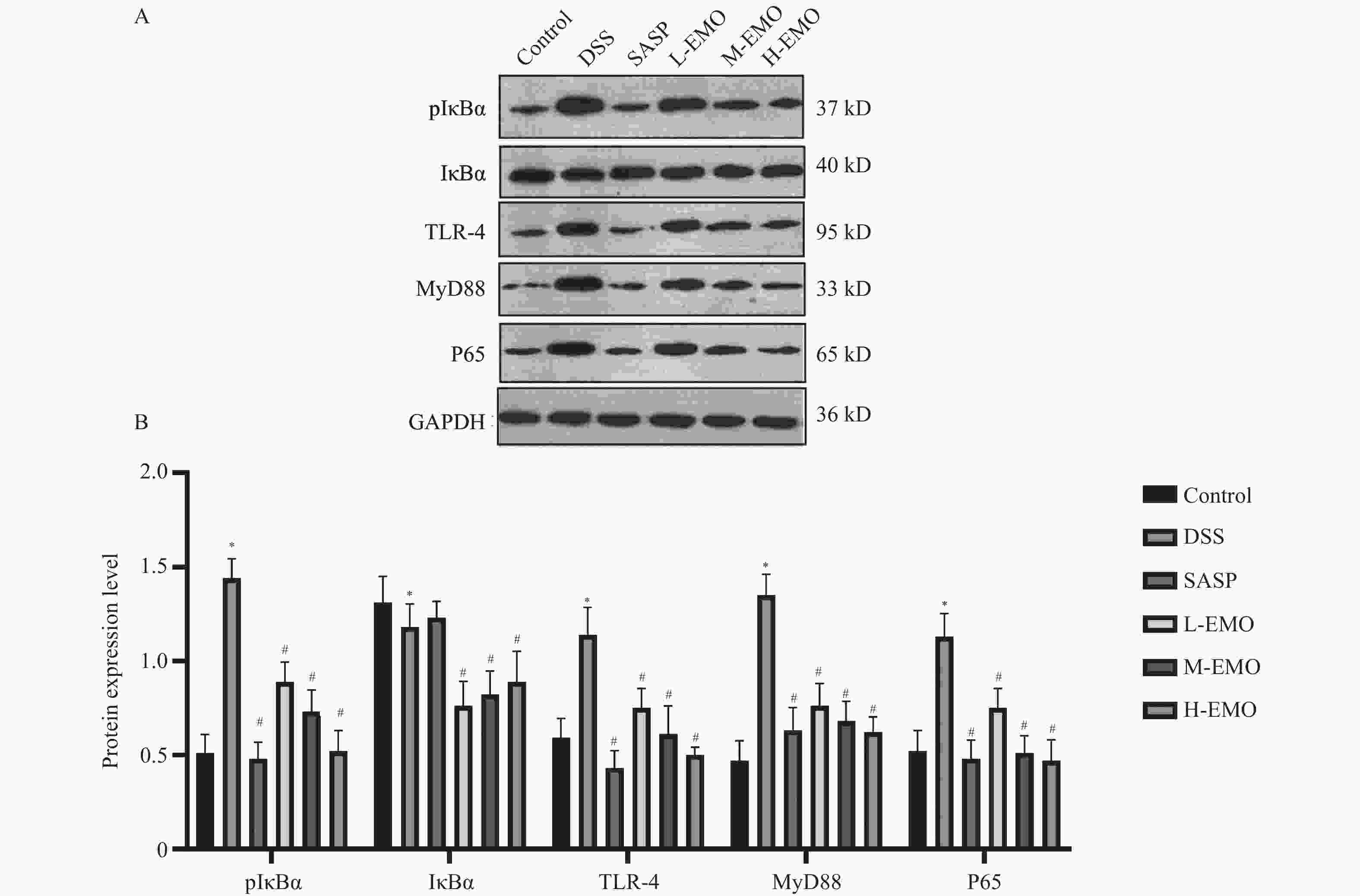
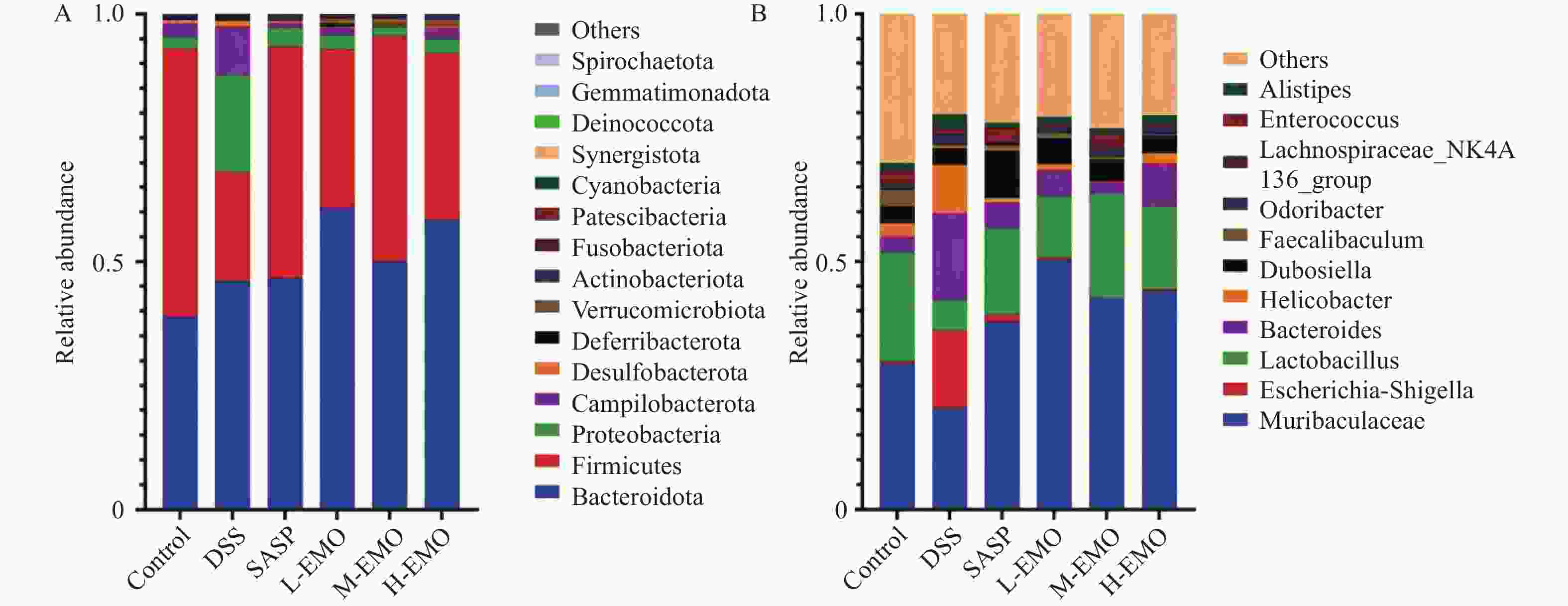
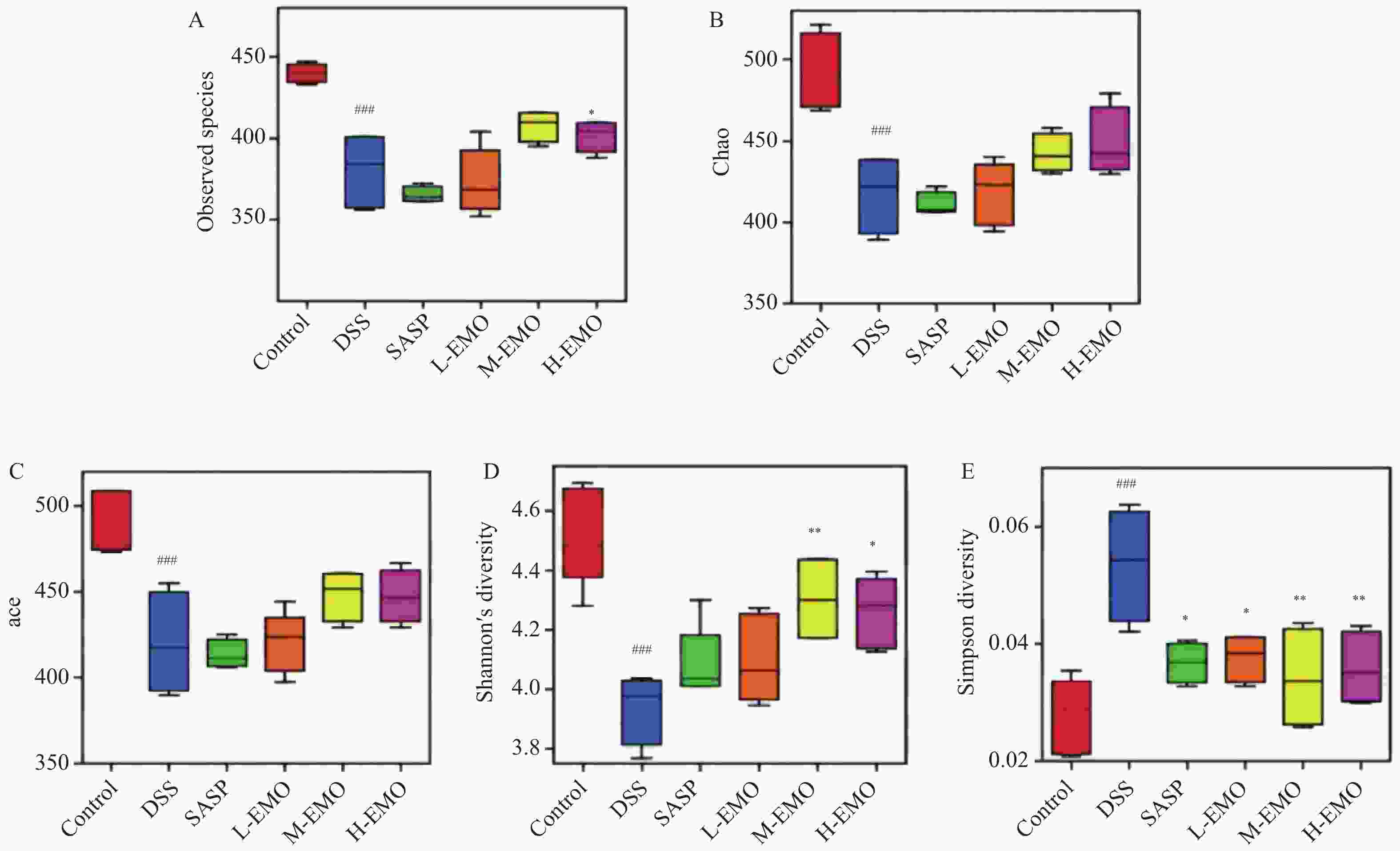
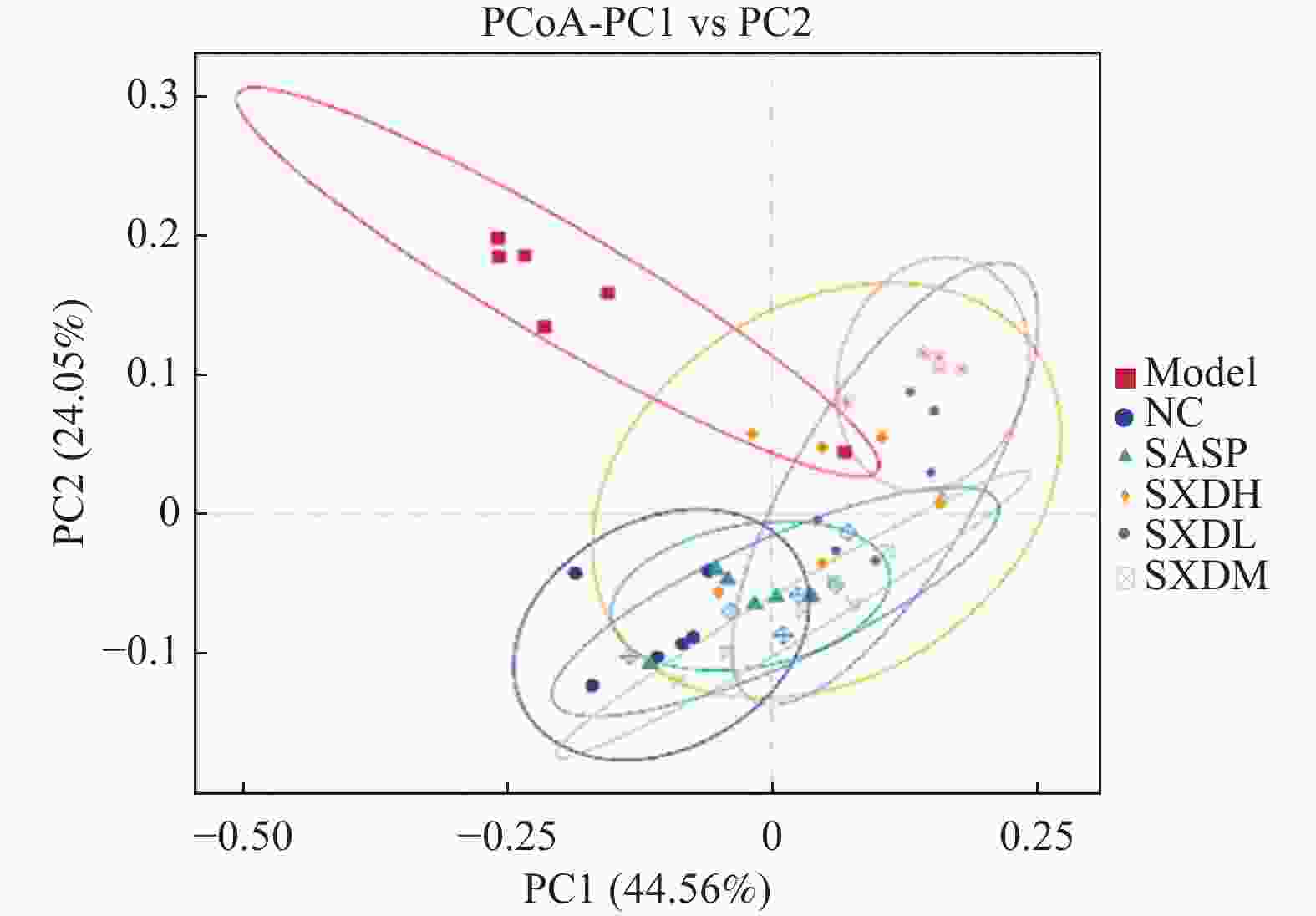
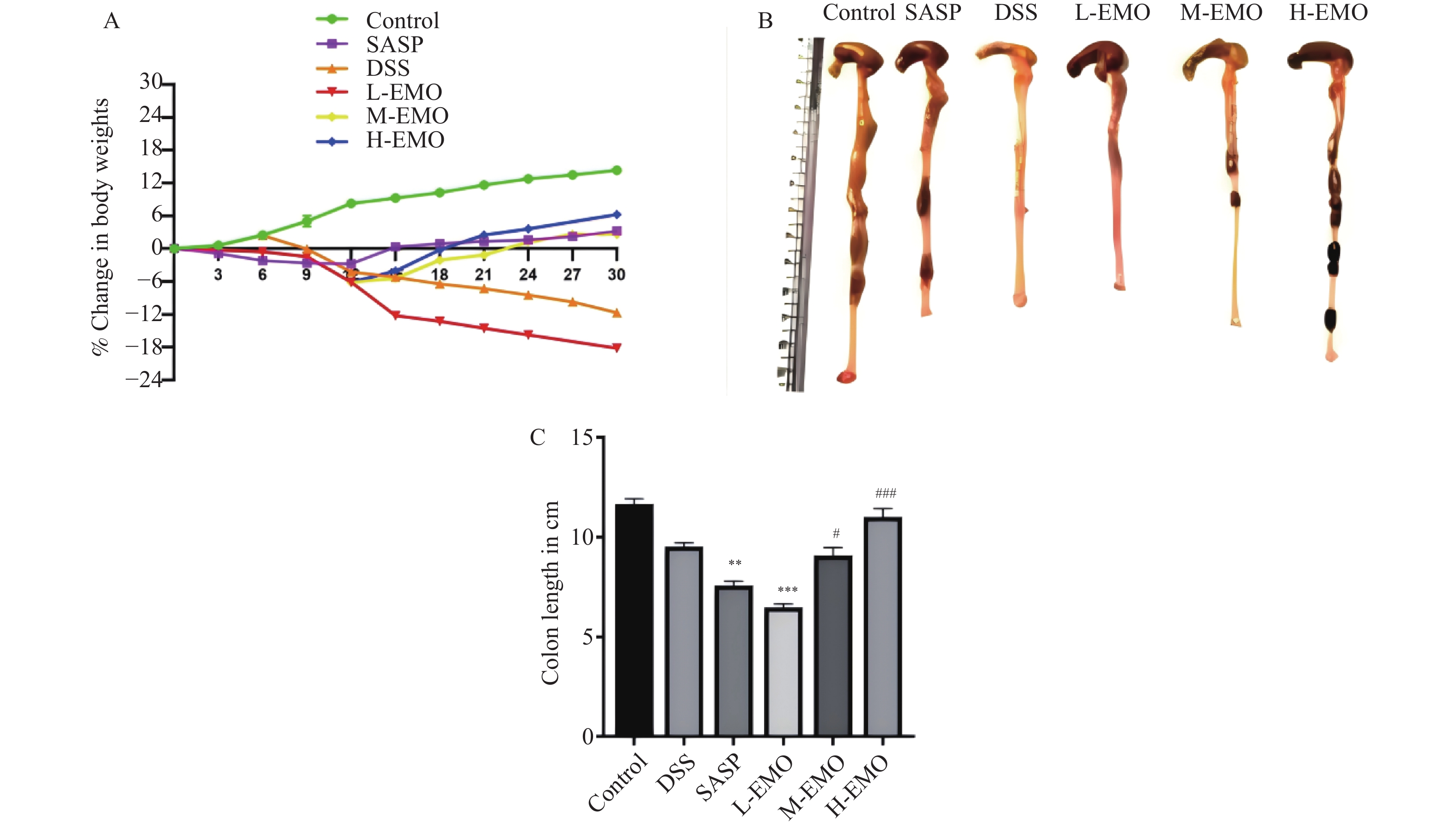
目的 探讨大黄素(emodin,EMO)对右旋糖酐硫酸钠(dextran sodium sulfate,DSS)诱导小鼠溃疡性结肠炎(ulcerative colitis,UC)的治疗作用及机制。 方法 DSS诱导UC小鼠模型,检测体重、结肠长度和组织病理学变化;酶联免疫吸附测定(enzyme-linked immunosorbent assay,ELISA)检测肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、白细胞介素-1β(interleukin-1β,IL-1β)、白细胞介素-6(interleukin-6,IL-6)、白细胞介素-10(interleukin-10,IL-10)及髓过氧化物酶(myeloperoxidase,MPO)的水平;通过蛋白质免疫印迹(Western blot)分析Toll样受体4(toll-like receptor 4,TLR4)/髓样分化因子88(myeloid differentiation factor 88,MyD88)/核因子κB(nuclear factor-kappa B,NF-κB)信号通路相关蛋白的表达;利用流式细胞术评估辅助性T细胞17(t helper 17,Th17)与调节性T细胞(regulatory t cell,Treg)的比例;16S rDNA测序评估肠道菌群组成。 结果 与正常组相比,DSS组小鼠体重显著下降、结肠长度缩短并出现明显组织学损伤(P < 0.001)。EMO干预尤其是高剂量组可剂量依赖性改善体重和结肠损伤(P < 0.05)。ELISA显示,EMO降低TNF-α、IL-1β、IL-6并升高IL-10水平(P < 0.05);Western blot提示EMO抑制TLR4/MyD88/NF-κB通路异常激活并恢复IκBα表达(P < 0.05);流式检测发现EMO降低Th17比例、升高Treg比例(P < 0.05);菌群分析显示EMO改善DSS诱导的菌群失调,增加有益菌相对丰度(P < 0.05)。 结论 EMO通过调控TLR4/MyD88/NF-κB通路、恢复Th17/Treg平衡及维持菌群稳态,有效改善DSS诱导的UC炎症反应和肠道损伤,为其作为UC潜在治疗药物提供理论依据。 -
关键词:
- 大黄素 /
- 右旋糖酐硫酸钠 /
- 溃疡性结肠炎 /
- TLR4/MyD88/NF-κB通路 /
- 肠道菌群 /
- Th17/Treg平衡
Abstract:Objective To investigate the therapeutic effects and mechanisms of emodin (EMO) on dextran sulfate (DSS)-induced ulcerative colitis (UC) in mice. Methods DSS induced UC mouse model, detection of body weight, colon length and histopathological changes. Enzyme-linked immunosorbent assay (ELISA) was used to measure tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), and myeloperoxidase (MPO) levels. Western blot analysis examined the expression of Toll-like receptor 4 (TLR4)/myeloid differentiation factor 88 (MyD88)/nuclear factor κB (NF-κB) signaling pathway-related proteins. Flow cytometry assessed the ratio of helper T cells 17 (Th17) to regulatory T cells (Treg). Additionally, 16S rDNA sequencing was employed to evaluate gut microbiota composition. Results Compared with the normal group, DSS-treated mice exhibited significant weight loss, shortened colon length, and marked histological damage (P < 0.001). EMO intervention, particularly at high doses, demonstrated dose-dependent improvements in body weight and colon injury (P < 0.05). ELISA analysis showed EMO reduced TNF-α, IL-1β, and IL-6 levels while increasing IL-10 (P < 0.05). Western blot results indicated EMO inhibited abnormal activation of the TLR4/MyD88/NF-κB pathway and restored IκB. Conclusion EMO effectively mitigates DSS-induced ulcerative colitis (UC) inflammation and intestinal damage by regulating the TLR4/MyD88/NF-κB pathway, restoring Th17/Treg balance, and maintaining microbial homeostasis, providing theoretical support for its potential as a UC therapeutic agent. -
Key words:
- Emodin /
- Dextran sulfate sodium /
- Ulcerative colitis /
- TLR4/MyD88/NF-κB pathway /
- Gut microbiota /
- Th17/Treg balance
-
表 1 各组Th17和Tregs细胞比例($\bar x \pm s $,%,n = 8)
Table 1. Proportions of Th17 and Treg cells in each group ($\bar x \pm s $,%,n = 8)
组别 Th17 Tregs Control 0.33 ± 0.03 4.17 ± 0.26 DSS 0.52 ± 0.03* 3.60 ± 0.29* SASP 0.81 ± 0.02* 2.19 ± 0.30* L-EMO 0.64 ± 0.02# 3.54 ± 0.27# M-EMO 0.53 ± 0.02# 3.74 ± 0.14# H-EMO 0.50 ± 0.04# 3.90 ± 0.24# F值 38.72 25.83 P值 <0.001 <0.001 注:与Control组相比,*P < 0.05;与DSS组相比,#P < 0.05。 表 2 各组小鼠肠道菌群在属水平的相对丰度变化($\bar x \pm s $,%,n = 8)
Table 2. Relative abundance of gut microbiota at the genus level in each group of mice ($\bar x \pm s $,%,n = 8)
组别 Muribaculaceae Muribaculaceae Lactobacillus Bacteroides Control 0.29 ± 0.05 0.005 ± 0.001 0.22 ± 0.03 0.030 ± 0.005 DSS 0.19 ± 0.03* 0.150 ± 0.020* 0.06 ± 0.02* 0.180 ± 0.030* SASP 0.38 ± 0.06# 0.015 ± 0.005# 0.17 ± 0.03# 0.061 ± 0.010# L-EMO 0.55 ± 0.08# 0.006 ± 0.003# 0.12 ± 0.02# 0.063 ± 0.011# M-EMO 0.45 ± 0.07# 0.006 ± 0.001# 0.21 ± 0.03# 0.025 ± 0.002# H-EMO 0.46 ± 0.07# 0.001 ± 0.001# 0.17 ± 0.03# 0.071 ± 0.015# F值 42.18 38.92 25.64 31.07 P值 <0.001 <0.001 <0.001 <0.001 与Control组相比,*P < 0.05;与DSS组相比,#P < 0.05。 -
[1] Singh S, Dulai P S. Ulcerative colitis: clinical manifestations and management[J]. Yamada's Textbook Gastroenterol, 2022: 1248-1293. [2] Kaluzna A, Olczyk P, Komosinska-vasse K. The role of innate and adaptive immune cells in the pathogenesis and development of the inflammatory response in ulcerative colitis[J]. J. Clin. Med, 2022, 11(2): 400 doi: 10.3390/jcm11020400 [3] Agrawal M, Allin K H, Petralia F, et al. Multiomics to elucidate inflammatory bowel disease risk factors and pathways[J]. Nat. Rev. Gastroenterol. Hepatol, 2022, 19(6): 399-409. doi: 10.1038/s41575-022-00593-y [4] Hu C, Liao S, Lv L, et al. Intestinal immune imbalance is an alarm in the development of IBD[J]. Mediators Inflamm, 2023, 2023(1): 1073984. [5] Zhang C, Yang Y. Targeting toll-like receptor 4 (TLR4) and the NLRP3 inflammasome: Novel and emerging therapeutic targets for hyperuricaemia nephropathy[J]. Biomol. Biomed, 2024, 24(4): 688. [6] Saleh H A, Yousef M H, Abdelnaser A. The anti-inflammatory properties of phytochemicals and their effects on epigenetic mechanisms involved in TLR4/NF-κB-mediated inflammation[J]. Front. Immunol, 2021, 12: 606069. doi: 10.3389/fimmu.2021.606069 [7] Zheng S, Xue T, Wang B, et al. Chinese medicine in the treatment of ulcerative colitis: the mechanisms of signaling pathway regulations[J]. Am. J. Chin. Med, 2022, 50(07): 1781-1798. doi: 10.1142/S0192415X22500756 [8] Guo M, Wang X. Pathological mechanism and targeted drugs of ulcerative colitis: A review[J]. Medicine (Baltimore), 2023, 102(37): e35020. [9] Yang Z, Lin S, Feng W, et al. A potential therapeutic target in traditional Chinese medicine for ulcerative colitis: Macrophage polarization[J]. Front. Pharmacol, 2022, 13: 999179. doi: 10.3389/fphar.2022.999179 [10] Wen Y, Yan P J, Fan P X, et al. The application of rhubarb concoctions in traditional Chinese medicine and its compounds, processing methods, pharmacology, toxicology and clinical research[J]. Front. Pharmacol, 2024, 15: 1442297. doi: 10.3389/fphar.2024.1442297 [11] Zeng Y, Liu X, Yi Q, et al. Free total rhubarb anthraquinones protect intestinal mucosal barrier of SAP rats via inhibiting the NLRP3/caspase-1/GSDMD pyroptotic pathway[J]. J. Ethnopharmacol, 2024, 326: 117873. doi: 10.1016/j.jep.2024.117873 [12] Du L, Hu X, Ren X, et al. Emodin Alleviates the Th17/Treg Imbalance by Targeting Myd88/NF-κB Signaling in Subacute Thyroiditis[J]. Rev. Bras. Farmacogn, 2023, 33(4): 847-855. doi: 10.1007/s43450-023-00368-9 [13] Lin Y, Xiong W, Xiao S, et al. Pharmacoproteomics reveals the mechanism of Chinese dragon's blood in regulating the RSK/TSC2/mTOR/ribosome pathway in alleviation of DSS-induced acute ulcerative colitis[J]. J Ethnopharmacol, 2020 Dec 5;263: 113221. [14] Yang C, Merlin D. Unveiling colitis: A journey through the dextran sodium sulfate-induced model[J]. Inflamm. Bowel Dis, 2024, 30(5): 844-853. doi: 10.1093/ibd/izad312 [15] Yu S, Qian H. Deoxyschizandrin treats mice with ulcerative colitis possibly via the TLR4/NF-κB signaling pathway[J]. Am. J. Transl. Res, 2021, 13(4): 3856. [16] Xu H, Zhu J, Lin X, et al. A comprehensive review of traditional chinese medicine in the management of ulcerative colitis[J]. Am. J. Chin. Med., 2025, 53(02): 435-473. doi: 10.1142/S0192415X2550017X [17] Yuan S, Wang Q, Li J, et al. Inflammatory bowel disease: an overview of Chinese herbal medicine formula-based treatment[J]. Chin. Med, 2022, 17(1): 74. doi: 10.1186/s13020-022-00633-4 [18] Tambovtseva R S, Arslan L A, Grigoryeva T A, et al. Narrative review on bacteria-derived metabolites in the pathogenesis of ulcerative colitis[J]. Clin. Microbiol. Rev, 2025: e00210-24. [19] Huang Q T, Ma X D, Zhang J N, et al. A hepatic oxidative metabolite of palmatine ameliorates DSS-induced ulcerative colitis by regulating macrophage polarization through AMPK/NF-κB pathway[J]. Am. J. Chin. Med, 2025, 53(01): 285-307. doi: 10.1142/S0192415X25500119 [20] Qin Z, Tang R, Liang J, et al. Berberine, a natural alkaloid: advances in its pharmacological effects and mechanisms in the treatment of autoimmune diseases[J]. Int. Immunopharmacol, 2024, 137: 112422. doi: 10.1016/j.intimp.2024.112422 [21] Yang F, Gap R, Luo X, et al. Berberine influences multiple diseases by modifying gut microbiota[J]. Front. Nutr, 2023, 10: 1187718. doi: 10.3389/fnut.2023.1187718 -





 下载:
下载: