Association between LOXL2 and Snail Expression Levels and Overall Survival Rate after Surgery in Patients with Cholangiocarcinoma
-
摘要:
目的 探讨赖氨酰氧化酶样蛋白2(LOXL2)及Snail在胆管癌(CCA)组织中的表达及其与患者临床病理特征和术后生存预后的关系。 方法 收集云南省第一人民医院2014年1月至2021年4月期间行手术切除的31例胆管癌病例及随访资料,应用免疫组织化学染色检测患者癌组织及癌旁正常组织标本中LOXL2及Snail的表达情况,分析2种蛋白表达与临床病理资料及预后的关系。 结果 肿瘤组织中LOXL2及Snail蛋白表达量均明显高于癌旁组织,且LOXL2表达与Snail表达存在着一定的正相关关系(r = 0.430,P = 0.016),Snail表达与淋巴结转移(P = 0.025)有关,与LOXL2及Snail高表达患者比较,LOXL2及Snail低表达患者术后总生存率较高(P < 0.05),单因素生存分析显示:Snail高表达、存在淋巴结转移与预后更差的胆管癌患者相关(P < 0.05)。Cox多因素生存分析结果表明:淋巴结转移可能是影响胆管癌患者预后的独立因素(P = 0.017)。 结论 LOXL2、Snail在胆管癌组织中表达增加,LOXL2与Snail 2种蛋白的表达密切相关,Snail的过表达可能更易出现淋巴结的转移,促进肿瘤细胞的侵袭性行为,并且淋巴结转移可能是影响胆管癌患者预后的独立因素。LOXL2及Snail过表达的患者其术后生存率相对较低。因此临床联合检测LOXL2及Snail对胆管细胞癌患者的预后判断具有重要意义。 Abstract:Objective To investigate the expression of lysoxygenase-like protein 2 (LOXL2) and Snail in the tissues of cholangiocarcinoma (CCA) and their association with clinicopathological features and postoperative survival. Methods 31 patients with cholangiocarcinoma who underwent surgical removal in the First People’s Hospital of Yunnan Province from 2014 to 2021 and their follow-up data were collected. The expression of LOXL2 and Snail in cancer tissues and adjacent normal tissues of patients was detected by immunohistochemical staining, and the association between the expression of the two proteins and clinicopathological data and prognosis was analyzed. Results The expression levels of LOXL2 and Snail in tumor tissues were significantly higher than those in adjacent tissues, and there was a positive correlation between LOXL2 expression and Snail expression (r = 0.430, P = 0.016). Snail expression was associated with lymph node metastasis (P = 0.025). Compared with patients with high expression of LOXL2 and Snail, patients with low expression of LOXL2 and Snail had higher overall survival rate (all P < 0.05). Univariate survival analysis showed that high expression of Snail and lymph node metastasis were associated with worse prognosis in patients with cholangiocarcinoma (all P < 0.05). Multivariate Cox survival analysis showed that lymph node metastasis might be an independent prognostic factor for patients with cholangiocarcinoma (P = 0.017). Conclusion The expression of LOXL2 and Snail is increased in cholangiocarcinoma tissues, and the expression of LOXL2 and Snail is closely related. The overexpression of Snail may be more prone to lymph node metastasis and promote the invasive behavior of tumor cells, and lymph node metastasis may be an independent factor affecting the prognosis of cholangiocarcinoma patients. Patients with LOXL2 and Snail overexpression have a relatively low postoperative survival rate.Therefore, the combined detection of LOXL2 and Snail is of great significance for the prognosis of patients with cholangiocarcinoma. -
Key words:
- Cholangiocarcinoma /
- LOXL2 /
- Snail /
- Invasive metastasis /
- Epithelial mesenchymal transition /
- Prognosis
-
胆管细胞癌(cholangiocarcinoma,CCA)是一种具有高度恶性的难治性肝胆系统肿瘤,起源于胆管上皮细胞,约占所有原发性肝胆肿瘤的15%[1]。尽管在大多数国家胆管癌被认为是一种发病率 < 6/100000的相对冷僻的疾病,但在全球范围内其发病率及死亡率不断上升[2-4]。CCA主要生物学特征是在癌症早期就有淋巴结和远处转移扩散的侵袭性行为,尽管通过近几十年的研究,对于CCA的认识、诊断及治疗有了很大程度提升,但在过去的10 a中,患者的预后并没有实质性的进展,5 a生存率(7%~20%)及术后复发率依然不尽人意[5-9]。近些年,在上皮起源的癌细胞侵袭转移过程中,观察到上皮细胞间连接、黏附的分解和细胞角蛋白丝的丢失,同时伴随着迁移功能的获得并产生大量细胞外基质成分,转化成间质细胞,细胞就可以离开其在上皮中的原始定位,从而向其它区域扩散,这一过程被命名为上皮-间质转化(epithelial-mesenchymal transition,EMT)[10]。其中Snail作为EMT过程中的关键转录因子,在体内通过抑制E-钙黏蛋白表达诱导上皮细胞发生EMT的表型变化,使肿瘤细胞在进展期间获得侵袭性和转移性[11]。最新的研究发现,LOXL2参与了Snail的基因表达及其所诱导的EMT的调控,因此LOXL2是Snail的上游调节因子。
本实验研究旨在了解LOXL2及Snail在胆管癌中的表达关系以及临床意义,分析LOXL2及Snail与临床病理特征的关系,初步探讨LOXL2及Snail在胆管癌的研究意义,为进一步在临床前及临床研究提供实验数据。
1. 资料与方法
1.1 一般资料
收集云南省第一人民医院于2014年1月至2021年4月肝胆外科收治并行根治性手术切除的胆管癌患者的病理组织标本31例。31例患者年龄46~76岁,平均年龄60.1岁,其中男性16例,女性15例。同时收集记录临床病理参数,包括肿瘤部位、肿瘤大小(以肿瘤最大直径表示)、淋巴结转移、肿瘤分化程度、患者性别、年龄、术后生存时间。31例患者在行根治性手术切除前均未接受放疗、化疗或其他辅助治疗。所有病例均经云南省第一人民医院病理科确诊为胆管癌。以癌旁正常胆管组织作对照。随访日期到2022年3月1日结束。
1.2 免疫组化
病理组织采用10%甲醛固定、脱水透明后石蜡包埋、制作3 μm厚组织切片,67 ℃烤片40 min,二甲苯溶液浸泡2次各15 min,依次不同浓度乙醇(100%、100%、95%、85%、75%)各2 min,抗原修复采用柠檬酸钠缓冲液(福州迈新生物,0.01 M pH6.0)高火加热3 min,后自然冷却至室温,3%过氧化氢(福州迈新生物)避光孵育15 min阻断内源性过氧化物酶,5%牛血清蛋白(德国Biofroxx公司)封闭非特异性抗原位点60 min,孵育一抗:单克隆鼠抗Snail+SLUG抗体(1∶500,英国Abcam公司,货号ab224731)4 ℃过夜;孵育一抗:多克隆兔抗LOXL2抗体(1∶100,英国Abcam公司,货号ab96233)4 ℃过夜,孵育二抗(抗小鼠/兔IgG聚合物,福州迈新生物,货号KIT-9921)18 min,显微镜下控制DAB显色(北京中杉金桥公司),苏木素复染,盐酸乙醇分化,温水浸泡反蓝,脱水,二甲苯透明,中性树胶(福州迈新生物)封片,正置荧光显微镜(德国ZEISS公司)下镜检采集数据。
1.3 免疫组化结果判定
评分方法参考既往研究[12-14]所述,对染色结果使用Image pro plus软件进行判定,综合2种蛋白的染色面积和强度分别进行评分,随后得分相加得到2种蛋白此次免疫组织化学染色结果。染色强度评判标准:棕褐色2分,棕黄色1分,淡黄色或无染色0分。染色面积评判标准:棕色面积 < 50%得分为0分,棕色面积 > 50%得分为1分。染色结果分别交给2名病理科医师评估,染色面积和染色强度分值相加,总分≥2分判定为高表达, < 2分为低表达。
1.4 统计学处理
使用SPSS 26.0软件进行统计学分析,2种蛋白表达强度相关性分析采用Spearman法,LOXL2、Snail蛋白表达强度与临床病理参数相关性分析使用Fisher确切概率法。绘制Kaplan-Meier总体生存曲线,Log-rank检验分析总体生存率差异,采用Cox回归模型进行多因素生存分析。P < 0.05为差异有统计学意义。
2. 结果
2.1 胆管癌病理组织中LOXL2和Snail表达及相关性
LOXL2表达集中在胞质和核周,以胞质为主,见图1;Snail表达集中在胞质及胞核,癌旁正常胆管组织未见2种蛋白表达,见图1。LOXL2高表达有16例,低表达有15例;Snail高表达有13例,低表达有18例。其中10例样本同时存在2种蛋白的高表达,12例同时存在2种蛋白的低表达。LOXL2蛋白的表达与Snail蛋白的表达存在着一定的正相关关系(r = 0.430,P = 0.016),差异具有统计学意义(P < 0.05),见表1。
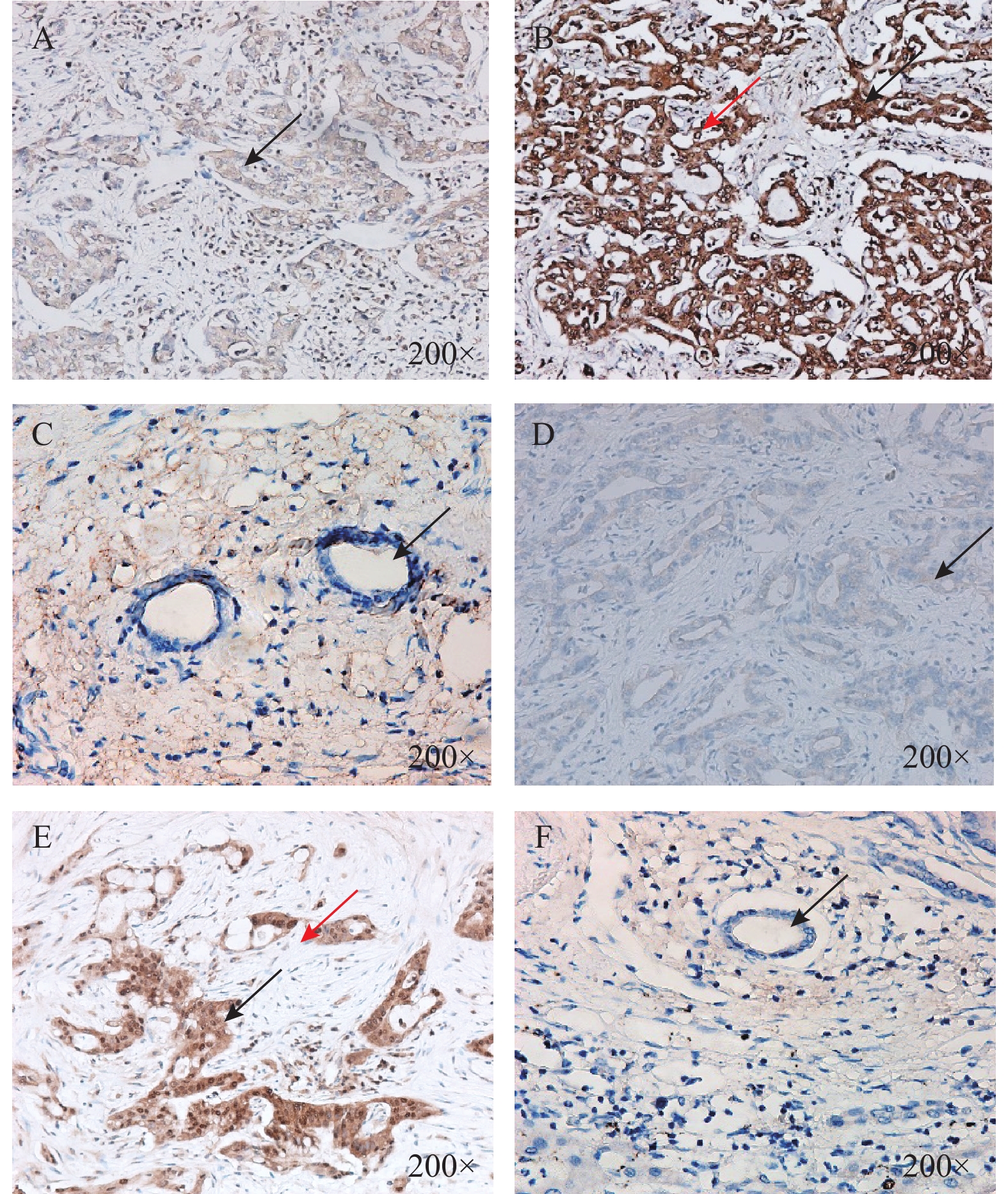 图 1 胆管癌病理组织和癌旁正常胆管组织LOXL2和Snail表达(×200)A:胆管癌组织中LOXL2低表达(箭头所示部位胞质);B:胆管癌组织中LOXL2高表达(黑色箭头所示部位胞质,红色箭头所示部位核周);C:癌旁正常胆管组织中LOXL2无表达(箭头所示癌旁正常胆管细胞)D:胆管癌组织中Snail低表达(箭头所示部位胞质);E:胆管癌组织中Snail高表达(黑色箭头所示部位胞质,红色箭头所示部位胞核);F:癌旁正常胆管组织中Snail无表达(箭头所示癌旁正常胆管细胞)。Figure 1. Expression of LOXL2 and Snail in pathological tissues and adjacent normal bile duct tissues of cholangiocarcinoma(×200)表 1 胆管癌病理组织中LOXL2和Snail表达相关性(n)Table 1. Correlation between LOXL2 and Snail expression in pathological tissues of cholangiocarcinoma (n)
图 1 胆管癌病理组织和癌旁正常胆管组织LOXL2和Snail表达(×200)A:胆管癌组织中LOXL2低表达(箭头所示部位胞质);B:胆管癌组织中LOXL2高表达(黑色箭头所示部位胞质,红色箭头所示部位核周);C:癌旁正常胆管组织中LOXL2无表达(箭头所示癌旁正常胆管细胞)D:胆管癌组织中Snail低表达(箭头所示部位胞质);E:胆管癌组织中Snail高表达(黑色箭头所示部位胞质,红色箭头所示部位胞核);F:癌旁正常胆管组织中Snail无表达(箭头所示癌旁正常胆管细胞)。Figure 1. Expression of LOXL2 and Snail in pathological tissues and adjacent normal bile duct tissues of cholangiocarcinoma(×200)表 1 胆管癌病理组织中LOXL2和Snail表达相关性(n)Table 1. Correlation between LOXL2 and Snail expression in pathological tissues of cholangiocarcinoma (n)表达情况 LOXL2 r P 高表达(n = 16) 低表达(n = 15) Snail 高表达(n = 13) 10 3 0.430 0.016* 低表达(n = 18) 6 12 *P < 0.05。 2.2 胆管癌病理组织中LOXL2和Snail表达与临床病理参数的关系
Snail高表达与胆管癌淋巴结转移存在密切联系(P = 0.025),差异具有统计学意义(P < 0.05),与其余临床病理参数均无显著相关性(P > 0.05);LOXL2表达与肿瘤大小、肿瘤位置、分化程度、淋巴结转移、患者性别、年龄等临床病理特征均无显著相关性(P > 0.05),见表2。
表 2 胆管癌病理组织中LOXL2和Snail表达与临床病理参数相关性[n(%)]Table 2. Correlation between LOXL2 and Snail expression and clinicopathological parameters in pathological tissues of cholangiocarcinoma [n(%)]临床病理参数 n Snail表达 P LOXL2表达 P 高表达 低表达 高表达 低表达 性别 男 16 6(37.5) 10(62.5) 0.722 9(56.3) 7(43.7) 0.724 女 15 7(46.7) 8(53.3) 7(46.7) 8(53.3) 年龄(岁) ≥60 16 8(50.0) 8(50.0) 0.473 11(68.7) 5(31.3) 0.076 < 60 15 5(33.3) 10(66.7) 5(33.3) 10(66.7) 肿瘤大小(cm) ≥5.7 17 6(35.3) 11(64.7) 0.481 7(41.2) 10(58.8) 0.285 < 5.7 14 7(50.0) 7(50.0) 9(64.3) 5(35.7) 肿瘤位置 肝内 26 10(38.5) 16(61.5) 0.625 13(50.0) 13(50.0) 1.000 肝外 5 3(60.0) 2(40.0) 3(60.0) 2(40.0) 肿瘤分化程度 高中 14 5(35.7) 9(64.3) 0.717 8(57.1) 6(42.9) 0.722 中低 17 8(47.1) 9(52.9) 8(47.1) 9(52.9) 淋巴结转移 有 15 10(66.7) 5(33.3) 0.025* 10(66.7) 5(33.3) 0.156 无 16 3(18.7) 13(81.3) 6(37.5) 10(62.5) *P < 0.05。 2.3 31例胆管癌患者生存分析
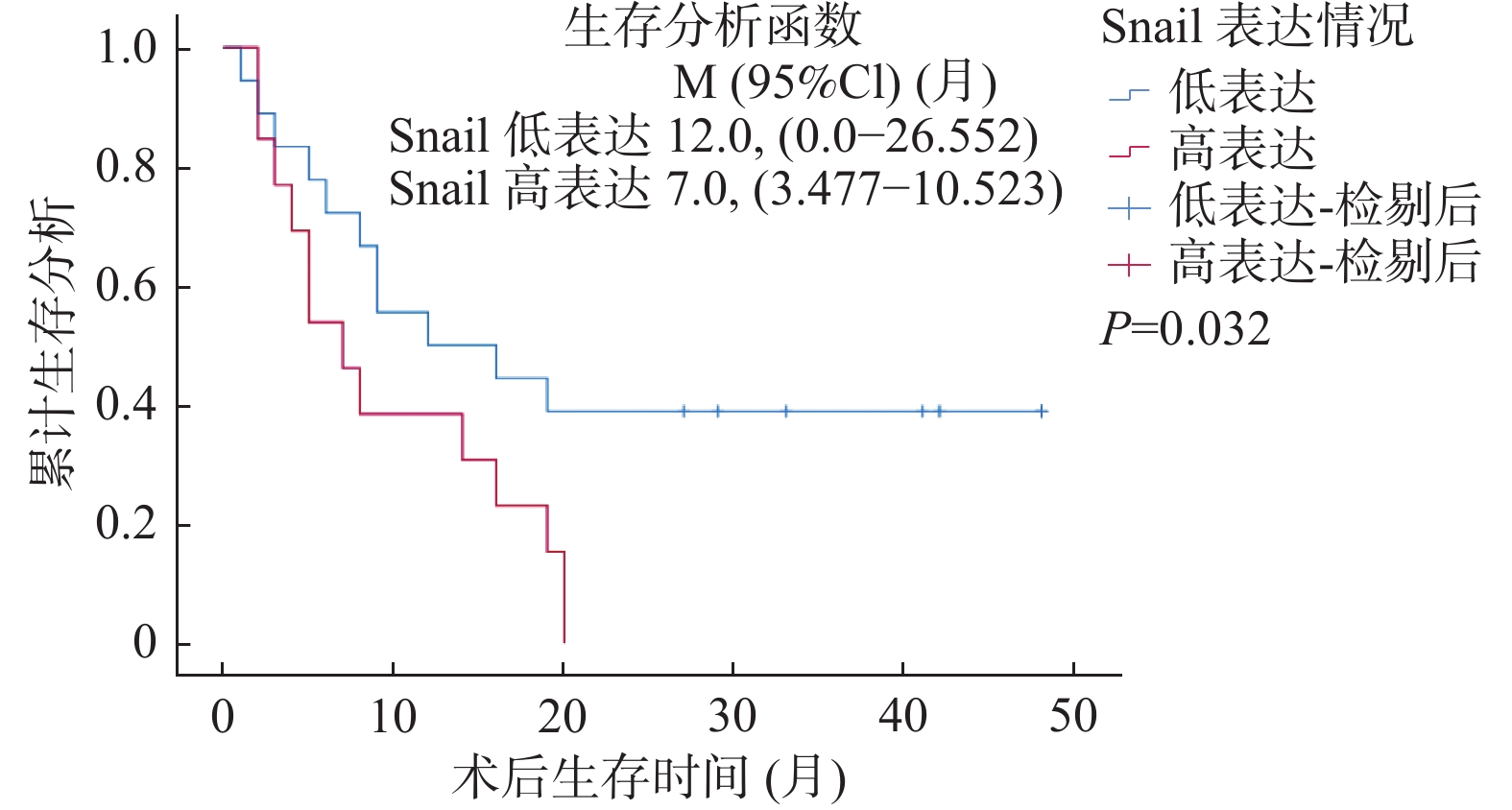
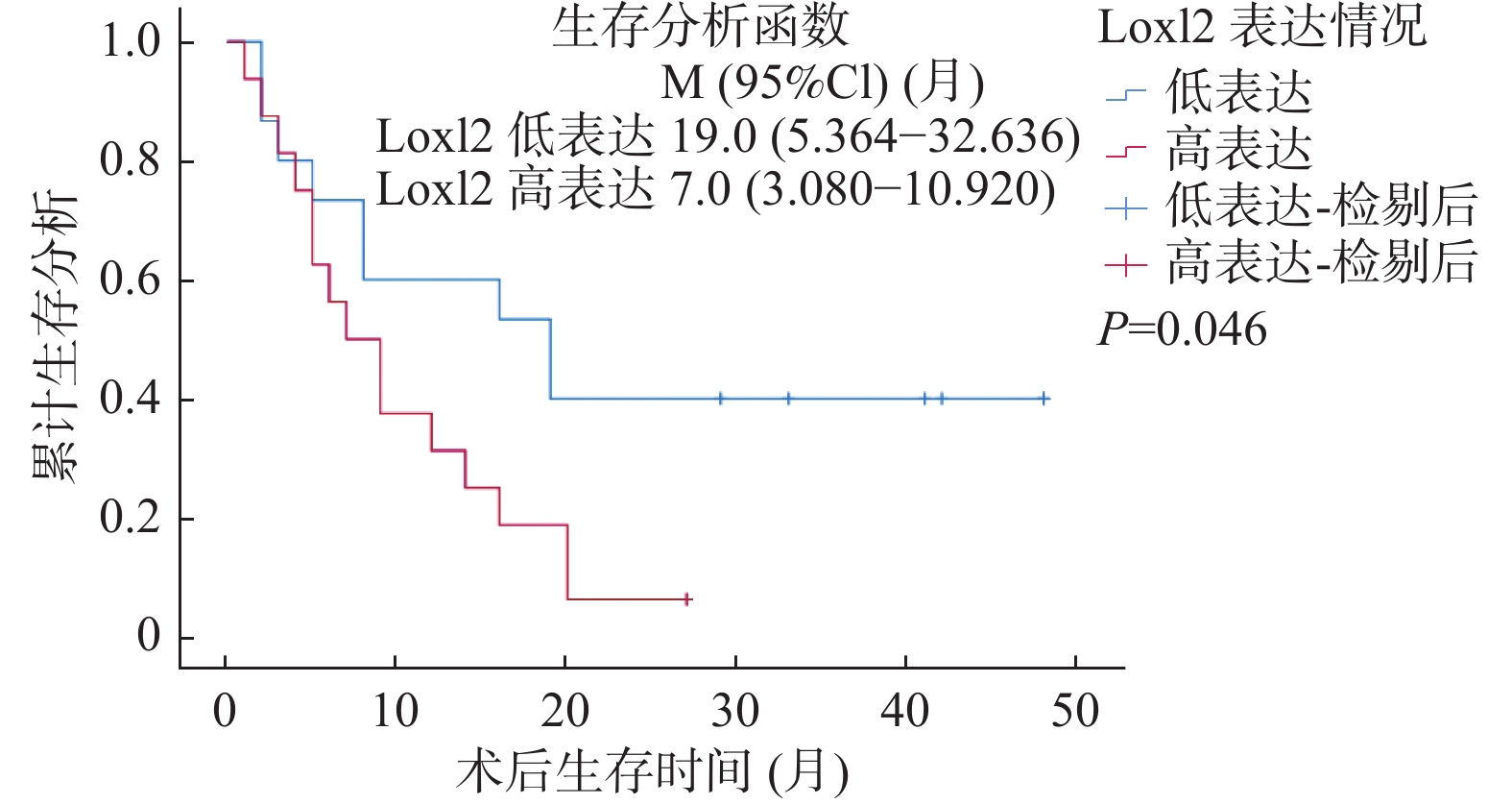
LOXL2与Snail免疫组化染色结果与生存结果分析表明,Snail高表达的病例术后总体生存率较低(LogRankP = 0.032),见图2,同时LOXL2高表达的病例术后总体生存率较低(LogRankP = 0.046),见图3。单因素总体生存率分析结果表明Snail高表达、有淋巴结转移与胆管癌患者更差的预后相关(P < 0.05);多因素Cox回归模型分析结果表明淋巴结转移可能是影响胆管癌患者预后的独立因素(P = 0.017),见表3。
表 3 胆管癌患者总生存率预后因素的单因素和多因素分析Table 3. Univariate and multivariate analysis of prognostic factors for overall survival of patients with cholangiocarcinoma
因数单因素分析 多因素分析 HR(95% CI) P HR(95% CI) P 性别 1.042(0.467~2.323) 0.920 - - 年龄 1.354(0.605~3.301) 0.461 - - 肿瘤大小 0.882(0.391~1.993) 0.764 - - 肿瘤位置 0.665(0.226~1.960) 0.460 - - 分化程度 0.724(0.319~1.643) 0.440 - - 淋巴转移 0.267(0.115~0.621) 0.002** 0.244(0.077~0.776) 0.017* Snail表达 0.437(0.194~0.983) 0.045* 1.135(0.377~3.420) 0.822 LOXL2表达 0.445(0.192~1.030) 0.059 - - *P < 0.05。 3. 讨论
由于胆管癌早期就表现出侵袭性行为,导致手术相关风险较大、根治性手术切除率低、术后复发率高以及较低的术后总生存率。因此了解胆管癌中导致侵袭性行为的相关机制是目前研究的一个重点,从而为预防癌症进展和复发提供一些新的治疗靶点,进而改善胆管癌的治疗效果。
2003年,G Akiri等[15]首次证明LOXL2可以促进肿瘤纤维化并增强乳腺癌的侵袭性。自那时以来,LOXL2因其在肿瘤发生发展中复杂且关键作用而引起了越来越多的关注。在许多癌症中观察到LOXL2异常高表达。以往的研究发现,在头颈部鳞状细胞癌[16]、乳腺癌[17]、肺癌[18]、结肠癌[19]、胃癌[20]、宫颈癌[21]、肝癌[22]和食管癌[23]中,LOXL2高表达与生存时间缩短和预后不良有关。LOXL2促进肿瘤的增殖、迁移、侵袭和转移,导致癌症的恶性进展[24-25]。LOXL2可以通过多种信号通路促进EMT并参与肿瘤微环境的组成,是肿瘤进展和转移的“帮凶”,其高表达是许多癌症患者的风险因素[26]。同时,高应鸿等[27]研究发现在48例胆管癌患者中,LOXL2的高表达与肿瘤的侵袭转移有关。D Bergeat等[28]通过对80例肝内胆管癌患者进行研究发现,LOXL2的高表达与不良预后有关,LOXL2高表达是影响肝内胆管癌患者总生存期和无病生存期的独立预后因素,并提出LOXL2有希望成为肝内胆管癌新的预后标志物和治疗靶点。在本研究中,LOXL2在胆管癌病理组织中呈现异常表达,然而其高表达与患者临床病理参数无明显相关性,这可能与本研究的样本容量小、免疫组织化学染色半定量分析结果准确性的差异有关,希望未来能有多中心、更大样本量以及更精细的实验设计进行更深入的研究。对胆管癌患者术后生存的随访分析显示,LOXL2高表达患者的术后生存率相对较低,提示在一定程度上LOXL2的表达可以预测胆管癌患者的预后。
Snail最早在黑腹果蝇中被发现[29],并被证明对中胚层的形成至关重要[30]。EMT可由不同的信号分子触发,如WNTs、成纤维细胞生长因子(fibroblast growth factor,FGF)、表皮生长因子(epidermal growth factor)、骨形态发生蛋白(bone morphogenetic protein,BMP)、转化生长因子β(transforming growth factor-β,TGF-β)、Notch、肝细胞生长因子(hepatocyte growth factor,HGF),并且这些信号分子都已被证实可以在不同的细胞环境中诱导Snail,从而使得肿瘤细胞获得侵袭性,向远处转移。过往研究表明,Snail在正常胆管细胞中不表达,但在胆管癌细胞中存在过表达情况,且其过表达促进肿瘤细胞侵袭、转移能力增强。康强等[14]对55例肝内胆管癌患者病理组织标本进行Snail蛋白免疫组织化学染色研究,结果提示肝内胆管癌患者病理组织标本Snail高表达与肿瘤的侵袭转移及不良预后有关,并认为Snail可以预测肝内胆管癌患者预后,同时也有望成为生物治疗的作用靶点;X Y Huang等[13]对140例肝内胆管细胞癌病理组织标本进行相关EMT指标的研究中,也得到了类似结果。在本次对31例胆管癌病理组织样本研究中,发现胆管癌患者病理组织中Snail呈现异常表达,并且其表达与临床病理参数相关。结果表明,Snail的高表达与侵袭转移性指标有关。对胆管癌患者术后生存的随访分析显示,Snail高表达的胆管癌患者的术后生存率相对较低。对多种变量进行胆管癌患者总生存期预后因素单因素和多因素分析的结果表明,Snail和侵袭性指标可作为预测胆管癌患者预后的指标。结合以往的研究,猜测胆管癌中Snail高表达可能会诱导肿瘤细胞EMT过程的进展,进而提高侵袭和转移能力。因此,笔者预测,对于Snail高表达的胆管癌患者,可以对Snail进行靶向沉默,从而减缓患者肿瘤远处转移和复发,这为胆管癌靶向药物的研究和开发提供了一个潜在位点。同时,对于胆管癌患者,术后对其病理组织常规行免疫组织化学染色,了解Snail表达情况,可对胆管癌患预后进行一定评估并为早期干预提供有力数据,对延长术后总体生存时间具有重要意义。
Snail在EMT过程中主要抑制E-钙黏蛋白的合成,其可通过Snail和Gfi(SNAG)结构域招募mSin3A协同加压复合物和组蛋白去乙酰化酶(HDAC),以抑制E-钙粘蛋白的表达[31],在这一过程中H Peinado等[32]发现LOXL2可以与SNAG结构域相互作用与Snail协同抑制E-钙黏蛋白,同时Snail介导的EMT依赖于Snail蛋白结构中完整K98和K137两个赖氨酸残基,LOXL2与K98和K137残基相互作用对维持Snail稳定性有着至关重要的作用。此外Snail的稳定性还受到GSK3β依赖性磷酸化和泛素化的精细控制,GSK3β以两种不同的基序磷酸化Snail,诱导其核输出并与β-Trcp结合,从而导致Snail降解,而LOXL2可以抵消这一过程,从而使Snail趋于稳定[33]。在喉癌和乳腺癌中,观察到LOXL2的过度表达促进其在内质网中的积累,并在内质网中与热休克蛋白5(HSPA5)相互作用,从而激活内质网应激反应的IRE1-XBP1信号通路。LOXL2依赖的IRE1-XBP1信号通路进而可以激活诱导几种EMT转录因子如Snail和ZEB的表达上调,进而有利于这些肿瘤的高侵袭性和转移[34]。在肝癌中发现LOXL2能够促进肿瘤通过Snail-FBP1轴上调HIF-1α/VEGF信号通路,促进肝癌的微血管生成和侵袭转移[35]。可以看出LOXL2通过细胞内和细胞外多种通路调节Snail蛋白的表达,然而在胆管癌中关于LOXL2与Snail表达相关性尚不明确,因此收集云南省第一人民医院胆管癌患者病理组织标本并对病理组织中LOXL2及Snail 2种蛋白表达进行免疫组化检测。在31例胆管癌病理组织标本中,研究结果表明,在胆管癌病理组织样本中LOXL2与Snail存在共表达情况,并且LOXL2的表达与Snail的表达存在一定的正相关关系,同时2种蛋白表达对患者术后总生存期的影响具有一定的相似性,因此推测二者在胆管癌中具有一定的协同性,此结果与以往在恶性肿瘤中的研究具有一定的相似性。但对于胆管癌中LOXL2与Snail表达调节涉及的具体方式及信号通路有待通过进一步的实验进行探讨和研究。
综上所述,LOXL2和Snail在胆管癌中呈现过表达,Snail过表达与胆管癌淋巴结转移有关,同时淋巴结转移是影响胆管癌患者预后的独立因素。在胆管癌中LOXL2和Snail表达存在一定的正相关关系。LOXL2和Snail过表达的胆管癌患者术后生存率相对较低,提示LOXL2和Snail可能是胆管癌患者预后的影响因素,有望成为生物治疗的新靶点。
-
图 1 胆管癌病理组织和癌旁正常胆管组织LOXL2和Snail表达(×200)
A:胆管癌组织中LOXL2低表达(箭头所示部位胞质);B:胆管癌组织中LOXL2高表达(黑色箭头所示部位胞质,红色箭头所示部位核周);C:癌旁正常胆管组织中LOXL2无表达(箭头所示癌旁正常胆管细胞)D:胆管癌组织中Snail低表达(箭头所示部位胞质);E:胆管癌组织中Snail高表达(黑色箭头所示部位胞质,红色箭头所示部位胞核);F:癌旁正常胆管组织中Snail无表达(箭头所示癌旁正常胆管细胞)。
Figure 1. Expression of LOXL2 and Snail in pathological tissues and adjacent normal bile duct tissues of cholangiocarcinoma(×200)
表 1 胆管癌病理组织中LOXL2和Snail表达相关性(n)
Table 1. Correlation between LOXL2 and Snail expression in pathological tissues of cholangiocarcinoma (n)
表达情况 LOXL2 r P 高表达(n = 16) 低表达(n = 15) Snail 高表达(n = 13) 10 3 0.430 0.016* 低表达(n = 18) 6 12 *P < 0.05。 表 2 胆管癌病理组织中LOXL2和Snail表达与临床病理参数相关性[n(%)]
Table 2. Correlation between LOXL2 and Snail expression and clinicopathological parameters in pathological tissues of cholangiocarcinoma [n(%)]
临床病理参数 n Snail表达 P LOXL2表达 P 高表达 低表达 高表达 低表达 性别 男 16 6(37.5) 10(62.5) 0.722 9(56.3) 7(43.7) 0.724 女 15 7(46.7) 8(53.3) 7(46.7) 8(53.3) 年龄(岁) ≥60 16 8(50.0) 8(50.0) 0.473 11(68.7) 5(31.3) 0.076 < 60 15 5(33.3) 10(66.7) 5(33.3) 10(66.7) 肿瘤大小(cm) ≥5.7 17 6(35.3) 11(64.7) 0.481 7(41.2) 10(58.8) 0.285 < 5.7 14 7(50.0) 7(50.0) 9(64.3) 5(35.7) 肿瘤位置 肝内 26 10(38.5) 16(61.5) 0.625 13(50.0) 13(50.0) 1.000 肝外 5 3(60.0) 2(40.0) 3(60.0) 2(40.0) 肿瘤分化程度 高中 14 5(35.7) 9(64.3) 0.717 8(57.1) 6(42.9) 0.722 中低 17 8(47.1) 9(52.9) 8(47.1) 9(52.9) 淋巴结转移 有 15 10(66.7) 5(33.3) 0.025* 10(66.7) 5(33.3) 0.156 无 16 3(18.7) 13(81.3) 6(37.5) 10(62.5) *P < 0.05。 表 3 胆管癌患者总生存率预后因素的单因素和多因素分析
Table 3. Univariate and multivariate analysis of prognostic factors for overall survival of patients with cholangiocarcinoma
因数单因素分析 多因素分析 HR(95% CI) P HR(95% CI) P 性别 1.042(0.467~2.323) 0.920 - - 年龄 1.354(0.605~3.301) 0.461 - - 肿瘤大小 0.882(0.391~1.993) 0.764 - - 肿瘤位置 0.665(0.226~1.960) 0.460 - - 分化程度 0.724(0.319~1.643) 0.440 - - 淋巴转移 0.267(0.115~0.621) 0.002** 0.244(0.077~0.776) 0.017* Snail表达 0.437(0.194~0.983) 0.045* 1.135(0.377~3.420) 0.822 LOXL2表达 0.445(0.192~1.030) 0.059 - - *P < 0.05。 -
[1] Banales J M,Cardinale V,Carpino G,et al. Expert consensus document:Cholangiocarcinoma:Current knowledge and future perspectives consensus statement from the european network for the study of cholangiocarcinoma (ENS-CCA)[J]. Nat Rev Gastroenterol Hepatol,2016,13(5):261-280. [2] Rizvi S,Khan S A,Hallemeier C L,et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies[J]. Nat Rev Clin Oncol,2018,15(2):95-111. [3] Bertuccio P,Malvezzi M,Carioli G,et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma[J]. J Hepatol,2019,71(1):104-114. doi: 10.1016/j.jhep.2019.03.013 [4] Banales J M,Marin J J G,Lamarca A,et al. Cholangiocarcinoma 2020:The next horizon in mechanisms and management[J]. Nat Rev Gastroenterol Hepatol,2020,17(9):557-588. doi: 10.1038/s41575-020-0310-z [5] Groot Koerkamp B,Wiggers J K,Allen P J,et al. Recurrence rate and pattern of perihilar cholangiocarcinoma after curative intent resection[J]. J Am Coll Surg,2015,221(6):1041-1049. doi: 10.1016/j.jamcollsurg.2015.09.005 [6] Lindnér P,Rizell M,Hafström L. The impact of changed strategies for patients with cholangiocarcinoma in this millenium[J]. HPB Surg,2015(12):1-6. [7] Spolverato G,Kim Y,Alexandrescu S,et al. Management and outcomes of patients with recurrent intrahepatic cholangiocarcinoma following previous curative-intent surgical resection[J]. Ann Surg Oncol,2016,23(1):235-243. [8] Strijker M,Belkouz A,Van Der Geest L G,et al. Treatment and survival of resected and unresected distal cholangiocarcinoma:A nationwide study[J]. Acta Oncol,2019,58(7):1048-1055. [9] Cambridge W A,Fairfield C,Powell J J,et al. Meta-analysis and meta-regression of survival after liver transplantation for unresectable perihilar cholangiocarcinoma[J]. Ann Surg,2021,273(2):240-250. doi: 10.1097/SLA.0000000000003801 [10] Thiery J P,Acloque H,Huang R Y,et al. Epithelial-mesenchymal transitions in development and disease[J]. Cell,2009,139(5):871-890. doi: 10.1016/j.cell.2009.11.007 [11] Nieto M A. The snail superfamily of zinc-finger transcription factors[J]. Nat Rev Mol Cell Biol,2002,3(3):155-166. [12] Ke A W,Shi G M,Zhou J,et al. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma[J]. Hepatology,2009,49(2):491-503. [13] Huang X Y,Zhang C,Cai J B,et al. Comprehensive multiple molecular profile of epithelial mesenchymal transition in intrahepatic cholangiocarcinoma patients[J]. PLoS One,2014,9(5):e96860. doi: 10.1371/journal.pone.0096860 [14] 康强,邹浩,刘立鑫,等. Snail在肝内胆管癌中的表达及其临床意义[J]. 中国普通外科杂志,2017,26(2):199-204. [15] Akiri G,Sabo E,Dafni H,et al. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo[J]. Cancer Res,2003,63(7):1657-1666. [16] Peinado H,Moreno-Bueno G,Hardisson D,et al. Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas[J]. Cancer Res,2008,68(12):4541-4550. doi: 10.1158/0008-5472.CAN-07-6345 [17] Ahn S G,Dong S M,Oshima A,et al. LOXL2 expression is associated with invasiveness and negatively influences survival in breast cancer patients[J]. Breast Cancer Res Treat,2013,141(1):89-99. [18] Zhan P,Lv X J,Ji Y N,et al. Increased lysyl oxidase-like 2 associates with a poor prognosis in non-small cell lung cancer[J]. Clin Respir J,2018,12(2):712-720. [19] Torres S,Garcia-Palmero I,Herrera M,et al. LOXL2 is highly expressed in cancer-associated fibroblasts and associates to poor colon cancer survival[J]. Clin Cancer Res,2015,21(21):4892-4902. doi: 10.1158/1078-0432.CCR-14-3096 [20] Peng L,Ran Y L,Hu H,et al. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway[J]. Carcinogenesis,2009,30(10):1660-1669. doi: 10.1093/carcin/bgp178 [21] Tian J,Sun H X,Li Y C,et al. LOXL 2 promotes the epithelial-mesenchymal transition and malignant progression of cervical cancer[J]. Onco Targets Ther,2019,12:8947-8954. [22] Choi J,Chung T,Rhee H,et al. Increased expression of the matrix-modifying enzyme Lysyl oxidase-like 2 in aggressive hepatocellular carcinoma with poor prognosis[J]. Gut Liver,2019,13(1):83-92. doi: 10.5009/gnl17569 [23] Li T Y,Xu L Y,Wu Z Y,et al. Reduced nuclear and ectopic cytoplasmic expression of lysyl oxidase-like 2 is associated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma[J]. Hum Pathol,2012,43(7):1068-1076. doi: 10.1016/j.humpath.2011.07.027 [24] Canesin G,Cuevas E P,Santos V,et al. Lysyl oxidase-like 2 (LOXL2) and E47 EMT factor:Novel partners in E-cadherin repression and early metastasis colonization[J]. Oncogene,2015,34(8):951-964. doi: 10.1038/onc.2014.23 [25] Hong X,Yu J J. Silencing of lysyl oxidase-like 2 inhibits the migration,invasion and epithelial-to-mesenchymal transition of renal cell carcinoma cells through the Src/FAK signaling pathway[J]. Int J Oncol,2019,54(5):1676-1690. [26] Wen B,Xu L Y,Li E M. LOXL2 in cancer:Regulation,downstream effectors and novel roles[J]. Biochim Biophys Acta Rev Cancer,2020,1874(2):188435. [27] 高应鸿,李天宇,高占峰,等. 胆管癌组织中赖氨酰氧化酶样蛋白-2的表达及其与EMT的关系[J]. 中华普通外科杂志,2008,10(23):784-787. [28] Bergeat D,Fautrel A,Turlin B,et al. Impact of stroma LOXL2 overexpression on the prognosis of intrahepatic cholangiocarcinoma[J]. J Surg Res,2016,203(2):441-450. doi: 10.1016/j.jss.2016.03.044 [29] Boulay J L,Dennefeld C,Alberga A. The Drosophila developmental gene snail encodes a protein with nucleic acid binding fingers[J]. Nature,1987,330(6146):395-398. [30] Alberga A,Boulay J L,Kempe E,et al. The snail gene required for mesoderm formation in Drosophila is expressed dynamically in derivatives of all three germ layers[J]. Development,1991,111(4):983-992. doi: 10.1242/dev.111.4.983 [31] Peinado H,Ballestar E,Esteller M,et al. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex[J]. Mol Cell Biol,2004,24(1):306-319. doi: 10.1128/MCB.24.1.306-319.2004 [32] Peinado H,Del Carmen Iglesias-De La Cruz M,Olmeda D,et al. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression[J]. Embo J,2005,24(19):3446-3458. doi: 10.1038/sj.emboj.7600781 [33] Peinado H,Portillo F,Cano A. Switching on-off Snail:LOXL2 versus GSK3beta[J]. Cell Cycle,2005,4(12):1749-1752. doi: 10.4161/cc.4.12.2224 [34] Cuevas E P,Eraso P,Mazón M J,et al. LOXL2 drives epithelial-mesenchymal transition via activation of IRE1-XBP1 signalling pathway[J]. Sci Rep,2017,7(1):44988. doi: 10.1038/srep44988 [35] Fan Z,Zheng W,Li H,et al. LOXL2 upregulates hypoxia-inducible factor-1α signaling through Snail-FBP1 axis in hepatocellular carcinoma cells[J]. Oncol Rep,2020,43(5):1641-1649. -






 下载:
下载:
 下载:
下载:

