Effect of Pristimerin on Proliferation of Oral Squamous Cell Carcinoma CAL-27 by Regulating Autophagy
-
摘要:
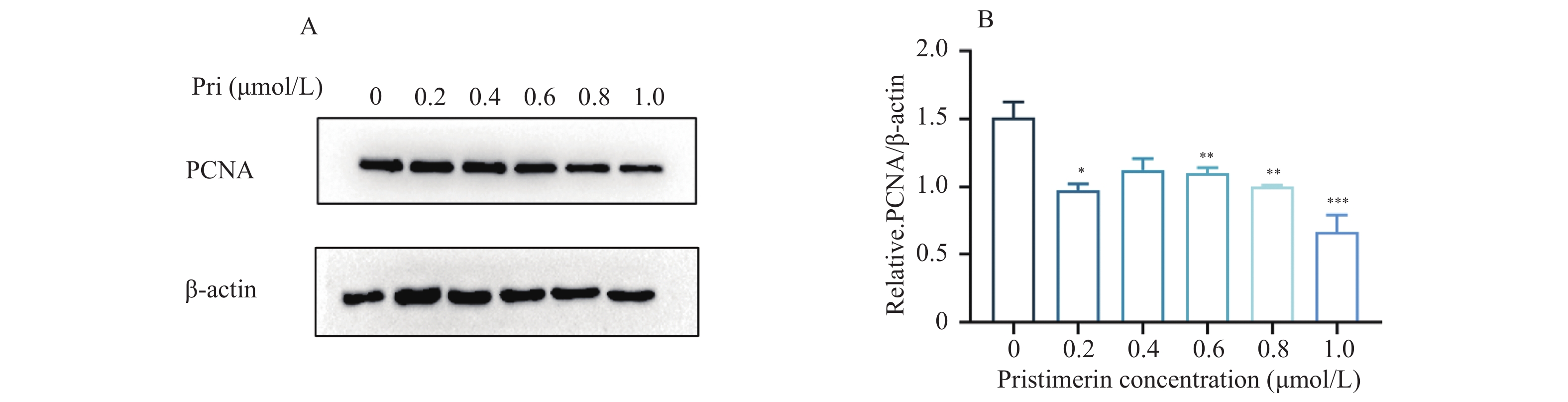
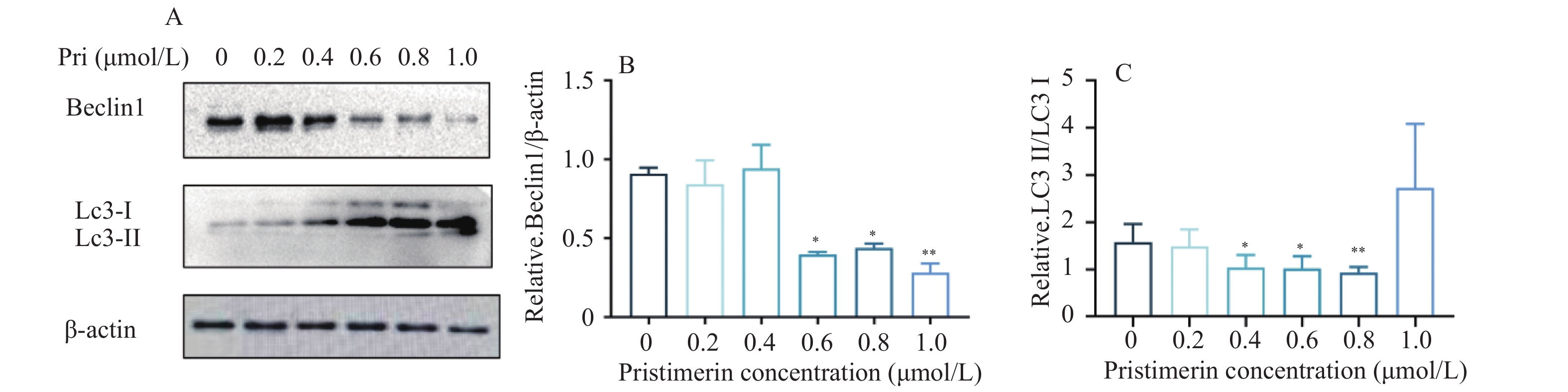
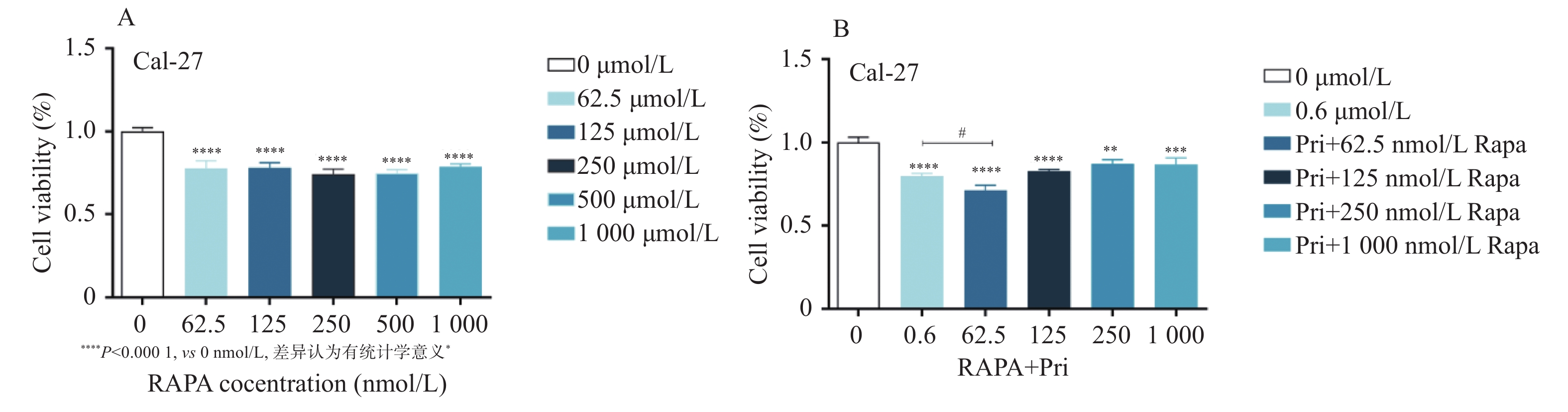
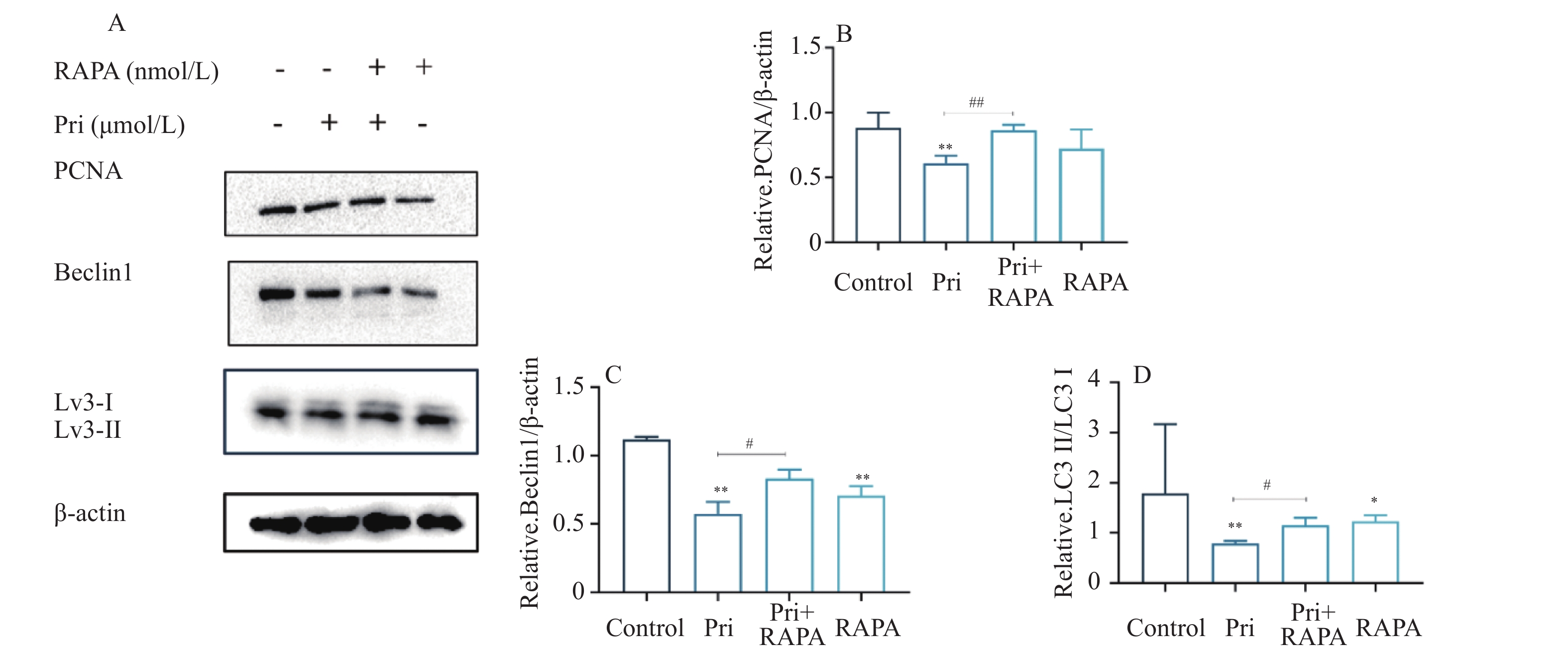
目的 探讨扁塑藤素对人口腔鳞状细胞癌细胞CAL-27增殖能力的影响及其相关作用机制。 方法 CCK8检测不同浓度的扁塑藤素处理CAL-27细胞后的增殖生物活性,计算不同作用时间段所对应的药物浓度水平(IC50);细胞集落形成实验检测CAL-27细胞克隆形成能力;蛋白质印迹法检测细胞增殖指标PCNA以及相关自噬通量BECLIN1、LC3B-II、LC3B-I蛋白表达水平;扁塑藤素联合自噬激动剂雷帕霉素(RAPA)处理CAL-27细胞,蛋白质印迹法检测PCNA以及相关自噬通量BECLIN1、LC3B-II、LC3B-I蛋白表达水平。 结果 较于空白组,各浓度梯度的扁塑藤素可抑制CAL-27细胞活力(P < 0.05),具有浓度-时间依耐性;相较于空白组,经扁塑藤素处理后细胞中PCNA、BECLIN1、LC3B-II/LC3B-I蛋白表达水平都出现明显上调(P < 0.05);扁塑藤素联合自噬激活剂雷帕霉素(RAPA)处理CAL-27细胞相较于仅使用扁塑藤素组细胞活性有所增强(P < 0.05),PCNA、BECLIN1、LC3B-II/LC3B-I蛋白的表达水平均出现明显上调(P < 0.05)。 结论 扁塑藤素在体外能够比较显著抑制口腔鳞状癌系CAL-27细胞的增殖,这可能与其下调自噬相关基因BECLIN1、LC3B-II、LC3B-I的表达相关。 Abstract:Objective To investigate the effect of pristimerin on the proliferation ability of human oral squamous cell carcinoma cells CAL-27 and its related mechanisms. Methods CCK-8 assay was performed to determine the proliferative bioactivity of CAL-27 cells treated with different concentrations of pristimerin, and the IC50 was calculated for different time periods. Colony formation assay was used to detect the cloning ability of CAL-27 cells. Western blotting was used to detect the protein expression levels of the proliferation marker PCNA and the autophagy flux markers BECLIN1, LC3B-II, and LC3B-I. CAL-27 cells were treated with pristimerin in combination with the autophagy inducer rapamycin (RAPA), and Western blotting was used to detect the protein expression levels of PCNA and the autophagy flux markers BECLIN1, LC3B-II, and LC3B-I. Results Compared with the blank group, the pristimerin at various concentration gradients can inhibit the viability of CAL-27 cells (P < 0.05), and it exhibits concentration-time dependence. Compared with the blank group, the expression levels of PCNA, BECLIN1 and LC3B-II/LC3B-I proteins in cells were significantly upregulated after treatment with the pristimerin (P < 0.05). Compared with the group treated with only the pristimerin, the combined treatment of the pristimerin with the autophagy activator rapamycin (RAPA) enhanced the cell activity of CAL-27 cells (P < 0.05), and the expression levels of PCNA, BECLIN1, and LC3B-II/LC3B-I proteins were significantly upregulated (P < 0.05). Conclusion Pristimerin significantly inhibits the proliferation of CAL-27 oral squamous carcinoma cells in vitro, which may be associated with downregulation of autophagy-related genes BECLIN1, LC3B-II, and LC3B-I. -
Key words:
- Pristimerin /
- Oral squamous cell carcinoma /
- Proliferation /
- Autophagy
-
维生素D不仅可以调节钙磷代谢和维护骨骼健康,还有很多骨骼以外的作用近来得到逐渐认知。大量研究证实,妊娠期妇女缺乏维生素D容易增加妊娠糖尿病、先兆子痫、早产的发病风险[1]。
妊娠期妇女的维生素D 含量水平过低同时会影响胎儿生长发育。维生素D会和胎盘母胎界面的维生素D受体结合后发挥作用,调控细胞增殖和分化[2],最终会影响胎盘生长和胎儿发育过程。
妊娠早期是胎儿器官分化的重要时期,胎儿四肢骨骼发育始于这个时期,了解此阶段妊娠妇女维生素D的水平对胎儿和母体都非常关键。维生素D经25羟化酶的催化后会合成25-羟维生素D(25OHD),它是体内维生素D的主要贮存形式,能反映出体内维生素D的营养状态[3]。本研究通过检测本院产检的妊娠早期妇女血清25OHD水平,旨为计划妊娠妇女合理补充维生素D提供建议。
1. 资料与方法
1.1 研究对象
选取2018年1月至2020年12月在昆明市妇幼保健院产检的妊娠早期(8~13周)妇女为研究对象,共4283名孕妇进入研究并进行25OHD检测,观测血清25OHD水平。按照年龄[4]分为16~20岁组,21~25岁组,26~30岁组,31~35岁组,36~40岁组,41~45岁组。
纳入标准[5]:(1)纳入研究妊娠期妇女均为孕早期,孕周 < 14周;(2)单胎妊娠且未检出出生缺陷;(3)本研究已通过昆明市妇幼保健院伦理委员会批准,入选者均签署知情同意书。
排除标准:(1)肝、肾及甲状腺疾病;(2)结核病、结缔组织病。
1.2 仪器与试剂
标本采集当天抽取研究对象空腹静脉血5 mL,经离心分离后当天使用iFlash3000-A全自动化学发光免疫分析仪(深圳亚辉龙)及厂家配套试剂盒测定孕妇血清总25OHD浓度。检测时严格按照说明书进行,样品符合检测标准,检测当日室内质控保证结果的准确性。
参照国内外标准[3],定义25OHD < 10 ng/mL(25 nmol/L)为维生素D严重缺乏,< 20 ng/mL(50 nmol/L)为缺乏,20~30 ng/mL(50~75 nmol/L)为不足,> 30 ng/mL(75 nmol/L)为充足。
1.3 统计学处理
采用SPSS 24.0统计学软件进行数据分析,计数资料以[n(%)]表示,组间比较采用χ2检验,计量资料以均值±标准差(
$\bar x \pm s $ )表示,采用单因素方差分析比较3组及以上队列,P < 0.05为差异有统计学意义。2. 结果
2.1 受试者25OHD状态分布
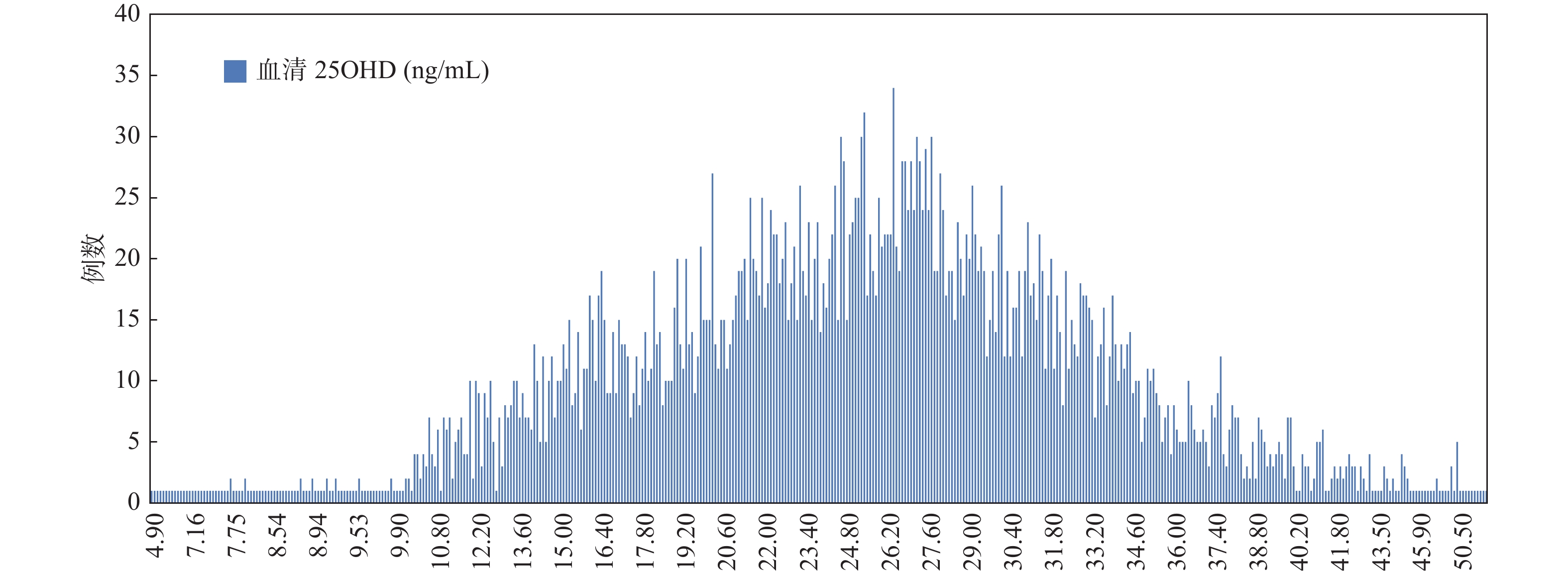
4 283名受试者血清25OHD呈近似正态分布,中位数为25.5 ng/mL,均值为(25.34±7.82)ng/mL,见图1。早孕期维生素D状况充足人群占27.20%,不足人群占48.28%,缺乏人群占22.18%,严重缺乏占2.33%,见表1。
表 1 受试者血清25OHD(ng/mL)的分布($\bar x \pm s $ )Table 1. Distribution of serum 25OHD (ng/ mL) in subjects ($\bar x \pm s $ )维生素D状况 25OHD(ng/mL) 受试者n(%) 充足 34.82 ± 4.36 1165(27.20) 不足 25.14 ± 2.75 2068(48.28) 缺乏 15.93 ± 2.66 950(22.18) 严重缺乏 8.45 ± 1.14 100(2.33) 2.2 不同年龄段间受试人群25OHD水平不足情况
将受试人群按16~20岁、21~25岁、26~30岁、31~35岁、36~40岁、41~45岁人群分层,6组人群平均25OHD水平均处于不足状态。以30 ng/mL为标准将受试者血清25OHD分为充足(> 30 ng/mL)、不充足(≤ 30 ng/mL)2队列,将各组人员频数进行卡方检验,结果显示各年龄段人群25OHD水平不充足人数之间,差异有有统计学意义(P < 0.05),16~20岁组血清25OHD ≤ 30 ng/mL人群数量最多,见表2。
表 2 不同年龄段受试者血清25OHD分布[n(%),($\bar x \pm s $ )]Table 2. 25OHD serum distribution of subjects of different ages [n(%),($\bar x \pm s $ )]组别(岁) ≤ 30 ng/mL > 30 ng/mL n 25OHD(ng/mL) χ2 P 16~20 81(83.51) 16(16.49) 97 23.11 ± 7.84 33.950 0.000* 21~25 764(76.48) 235(23.52) 999 24.60 ± 7.60 26~30 1391(73.44) 503(25.56) 1894 25.34 ± 7.71 31~35 659(70.86) 271(29.14) 930 26.04 ± 7.75 36~40 202(66.67) 101(33.33) 303 25.85 ± 8.82 41~45 31(51.67) 29(48.33) 60 27.73 ± 8.41 *P < 0.05。 2.3 不同年龄段间受试人群25OHD水平比较
对受试人年龄和血清25OHD水平进行相关分析,发现妊娠年龄与血清25OHD水平有相关性(r = 0.073,P < 0.01)。6个年龄段间比较,31~35岁组血清25OHD水平要高于16~20岁组,差异有统计学意义(P < 0.05)。31~35岁组血清25OHD水平高于21~25岁组,差异有统计学意义(P < 0.05),其余组间比较血清25OHD水平,差异无统计学意义(P > 0.05),见表3。
表 3 不同年龄段受试者血清25OHD水平比较Table 3. Comparison of serum 25OHD levels among subjects of different ages分组
(岁)比较组
(岁)P 95% CI 下限 上限 16~20 21~25 0.696 −3.9762 0.9991 26~30 0.107 −4.6686 0.2189 31~35 0.010* −5.4235 −0.4264 36~40 0.064 −5.5468 0.0771 41~45 0.122 −8.6406 −0.5969 21~25 26~30 0.188 −1.6122 0.1396 31~35 0.001* −2.4621 −0.4108 36~40 0.353 −2.9172 0.4246 41~45 0.092 −6.5086 0.2481 26~30 31~35 0.304 −1.6080 0.2078 36~40 0.998 −2.1122 1.0922 41~45 0.398 −5.7415 0.9537 31~35 36~40 1.000 −1.4975 1.8777 41~45 0.883 −5.0799 1.6922 36~40 41~45 0.854 −5.4980 1.7302 *P < 0.05。 3. 讨论
维生素D近年来作用被逐渐认知[6-13],妊娠期妇女的维生素D水平与新生儿维生素D水平相关[14],母体维生素D水平会同时影响母婴双方的健康[15-20],高水平维生素D能够增加受精卵的着床成功率,同时促进胎儿的免疫系统以及骨骼的生长发育;维生素D含量低会增加妊娠期妇女先兆子痫发生风险,同时会使得新生儿出现低体重的风险增加,严重时甚至会导致新生儿肾脏不能正常发育[21-22]。
在该次研究中发现,2020年受试者人均血清25OHD值高于2019年、2018年受试人群水平(P < 0.05),可能与近年来妊娠期妇女越来越重视孕期营养保健补充有关。
4 283名受试者血清25OHD水平达到充足的只有27.20%(1165/4283),其它孕妇维生素D含量均属于不足、缺乏甚至严重缺乏状态。平均25OHD水平为(25.34±7.82)ng/mL,说明近3 a来,虽然妊娠期妇女血清25OHD水平逐年提高,但平均水平未达到充足状态。增加日照和选择富含维生素D食物摄入是预防维生素D缺乏的有效措施[3],多脂肪鱼类便是一种有效食物,但多脂肪鱼类不是昆明地区常规饮食,且妊娠期维生素D需求较非孕期增加,这可能是本地区妊娠期妇女维生素D缺乏的原因之一。建议妊娠期妇女增加户外活动,摄入富含维生素D食物,根据血清25OHD检测结果摄入维生素D补充剂以满足机体对维生素D的需求。《中国居民膳食指南》[23]建议妊娠期妇女维生素D推荐摄入量为400 IU/d,《维生素D及其类似物临床应用共识》[3]建议妊娠和哺乳期妇女补充维生素D 1500~2000 IU/d,《维生素矿物质补充剂在保持孕期妇女和胎儿健康中的应用:专家共识》[24]建议孕期妇女每日摄入维生素D 200 IU/d,冬季补充400 IU/d。各指南、共识推荐孕妇摄入维生素D剂量有较大的差异,妊娠期妇女维生素D补充剂量摄入量需进一步研究确定。
妊娠年龄与血清25OHD水平有相关性,31~35岁组血清OHD水平高于16~20岁组、21~25岁组。究其原因,16~25岁本身处于生长发育旺盛阶段,维生素D需求较大,在怀孕后,胎儿对钙和维生素D的需求不断增加,从而加重母体维生素D含量缺乏情况。建议针对低龄妊娠妇女开展血清25OHD检测工作,及时筛查高危孕妇并进行干预。本研究显示31~35岁组血清25OHD水平较高,但有关文献报道[25],与适龄产妇(25~29岁)相比,30~34岁组发生出生缺陷儿风险较高。高龄、低龄妊娠各有弊端[26],故应提倡适龄妊娠,对于高危孕妇要提供更完善的孕产期保健服务,以便获得更好的母儿预后。
-
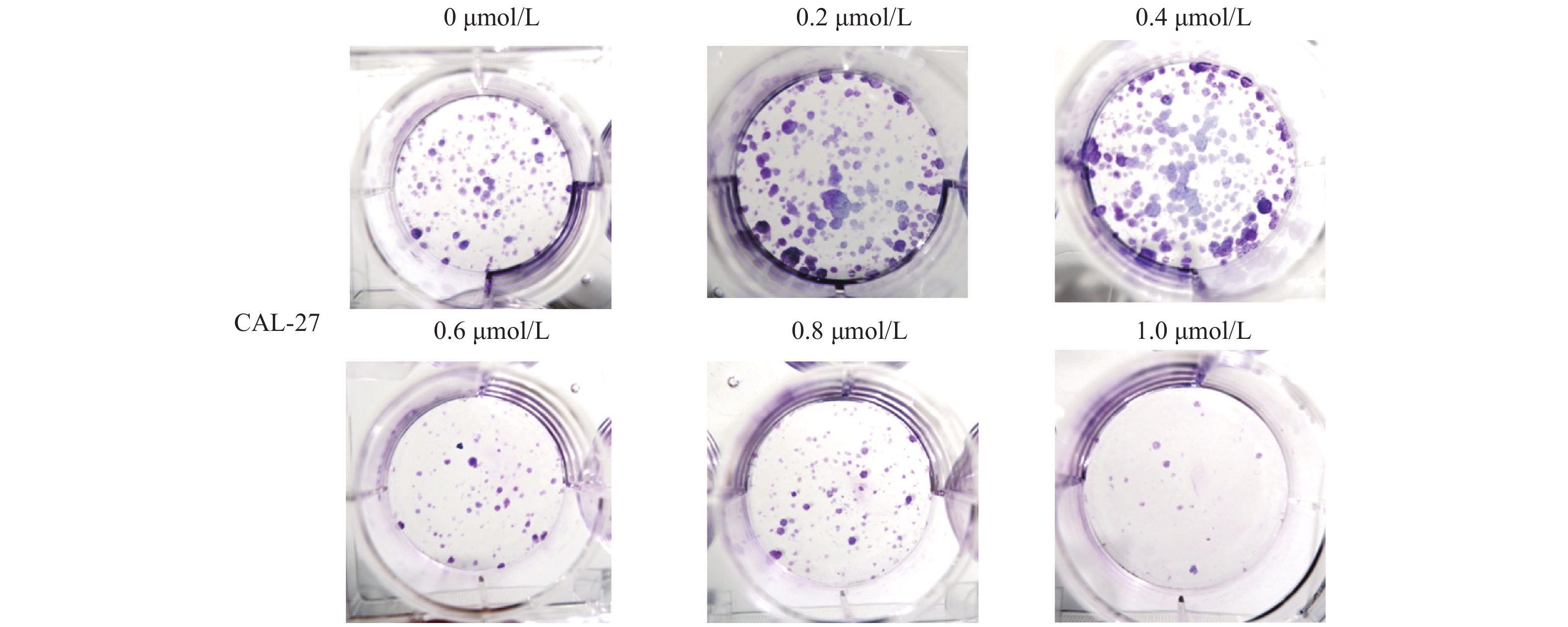
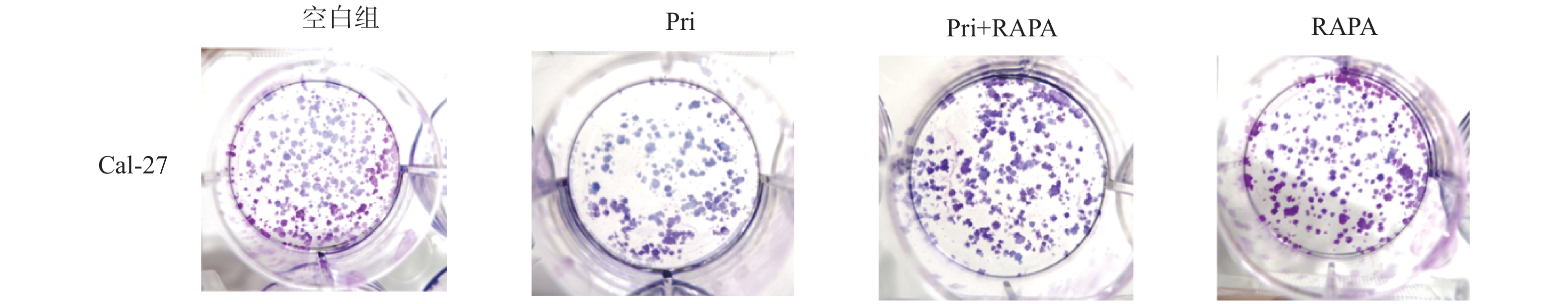
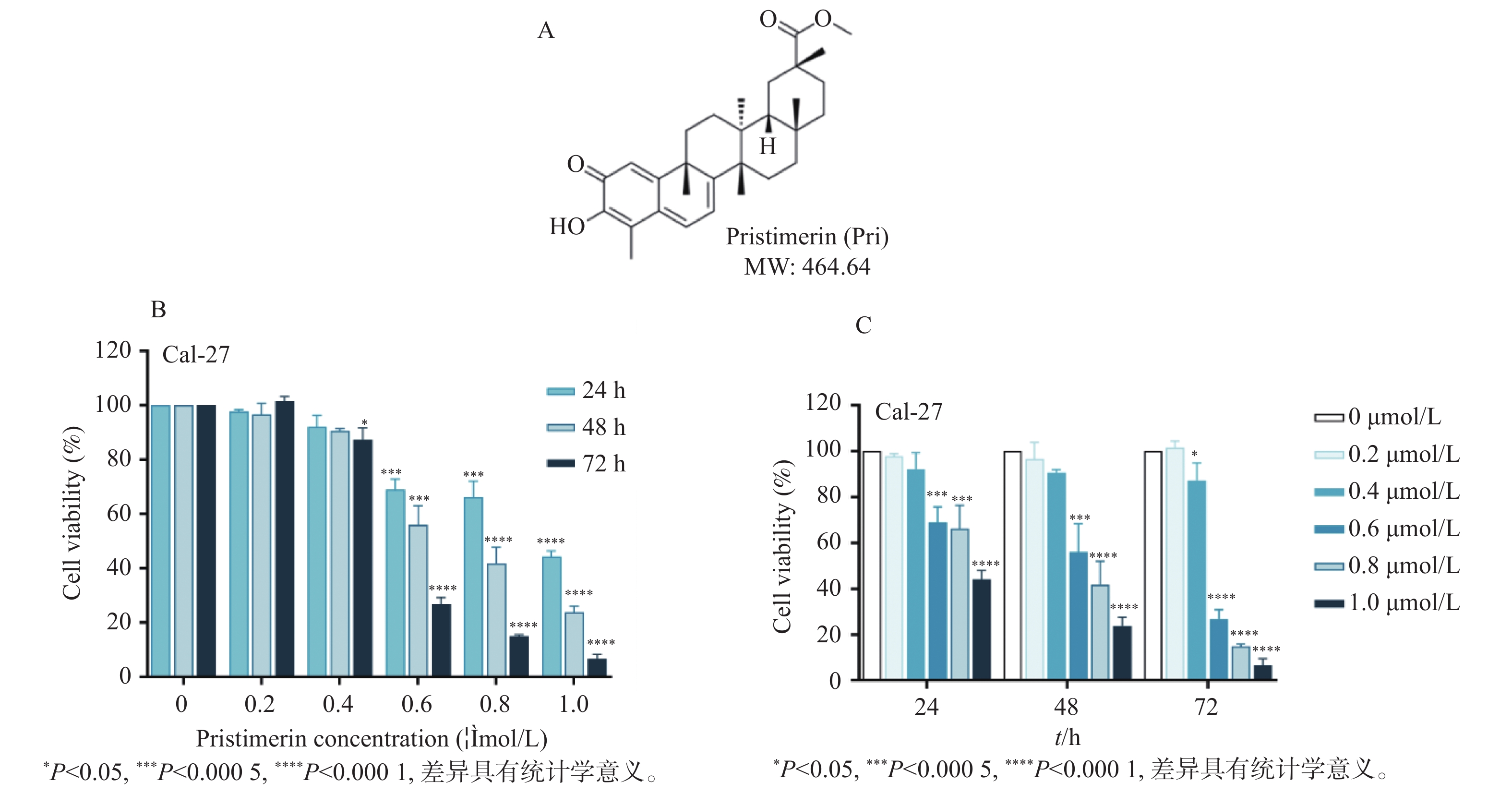
图 1 扁塑藤素抑制口腔鳞状癌Cal-27细胞增殖
A:扁塑藤素化学式和分子结构[17];B:CCK-8测定不同浓度扁塑藤素处理Cal-27细胞对细胞增殖的影响;C:CCK-8定扁塑藤素处理Cal-27细胞24 h、48 h、72 h对细胞增殖的影响。与0 μmol/L组相比,*P < 0.05,***P < 0.0005,****P < 0.0001。
Figure 1. Pristimerin suppresses proliferation of oral squamous cell carcinoma cell
-
[1] Liu J,Jiang X,Zou A,et al. CircIGHG-induced epithelial-to-mesenchymal transition promotes oral squamous cell carcinoma progression via miR-142-5p/igf2bp3 signaling[J]. Cancer Res,2021,81(2):344-355. doi: 10.1158/0008-5472.CAN-20-0554 [2] Sung H,Ferlay J,Siegel R L,et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2021,71(3):209-249. doi: 10.3322/caac.21660 [3] Yang Z,Yan G,Zheng L,et al. YKT6,as a potential predictor of prognosis and immunotherapy response for oral squamous cell carcinoma,is related to cell invasion,metastasis,and CD8+ T cell infiltration[J]. Oncoimmunology,2021,10(1):1938890. doi: 10.1080/2162402X.2021.1938890 [4] Ling Z,Cheng B,Tao X. Epithelial-to-mesenchymal transition in oral squamous cell carcinoma: Challenges and opportunities[J]. Int J Cancer,2021,148(7):1548-1561. doi: 10.1002/ijc.33352 [5] Khadela A,Shah Y,Mistry P,et al. Immunomodulatory therapy in head and neck squamous cell carcinoma: Recent advances and clinical prospects[J]. Technol Cancer Res Treat,2023,22:2081074849. [6] Debnath J,Gammoh N,Ryan K M. Autophagy and autophagy-related pathways in cancer[J]. Nat Rev Mol Cell Biol,2023,24(8):560-575. doi: 10.1038/s41580-023-00585-z [7] Cheon S Y,Kim H,Rubinsztein D C,et al. Autophagy,cellular aging and age-related human diseases[J]. Exp Neurobiol,2019,28(6):643-657. doi: 10.5607/en.2019.28.6.643 [8] Lock R,Kenific C M,Leidal A M,et al. Autophagy-dependent production of secreted factors facilitates oncogenic ras-driven invasion[J]. Cancer Discov,2014,4(4):466-479. doi: 10.1158/2159-8290.CD-13-0841 [9] Tompkins K D,Thorburn A. Regulation of apoptosis by autophagy to enhance cancer therapy[J]. Yale J Biol Med,2019,92(4):707-718. [10] Bahrami A,Khazaei M,Hassanian S M,et al. Targeting the tumor microenvironment as a potential therapeutic approach in colorectal cancer: rational and progress[J]. J Cell Physiol,2018,233(4):2928-2936. doi: 10.1002/jcp.26041 [11] Wang Y,Feng W,Wang X,et al. The multifaceted mechanisms of pristimerin in the treatment of tumors state-of-the-art[J]. Biomed Pharmacother,2022,154:113575. doi: 10.1016/j.biopha.2022.113575 [12] Shaaban A A,El-Kashef D H,Hamed M F,et al. Protective effect of pristimerin against lps-induced acute lung injury in mice[J]. Int Immunopharmacol,2018,59:31-39. doi: 10.1016/j.intimp.2018.03.033 [13] Cheng S,Zhang Z,Hu C,et al. Pristimerin suppressed breast cancer progression via mir-542-5p/dub3 axis[J]. Onco Targets Ther,2020,13(1171):6651-6660. [14] Li J,Guo Q,Lei X,et al. Pristimerin induces apoptosis and inhibits proliferation,migration in h1299 lung cancer cells[J]. J Cancer,2020,11(21):6348-6355. doi: 10.7150/jca.44431 [15] Zhao Q,Bi Y,Guo J,et al. Effect of pristimerin on apoptosis through activation of ros/ endoplasmic reticulum (ER) stress-mediated noxa in colorectal cancer[J]. Phytomedicine,2021,80:153399. doi: 10.1016/j.phymed.2020.153399 [16] Zhang Y,Wang J,Hui B,et al. Pristimerin enhances the effect of cisplatin by inhibiting the miR-23a/Akt/GSK3β signaling pathway and suppressing autophagy in lung cancer cells[J]. Int J Mol Med,2019,43(3):1382-1394. [17] Zhao Q,Cheng X,Yu W,et al. Pristimerin induces apoptosis and tumor inhibition of oral squamous cell carcinoma through activating ros-dependent er stress/noxa pathway[J]. Phytomedicine,2021,92(11):153723. [18] Zhao Q,Liu Y,Zhong J,et al. Pristimerin induces apoptosis and autophagy via activation of ros/ask1/jnk pathway in human breast cancer in vitro and in vivo[J]. Cell Death Discov,2019,5(87):125. [19] 石艺,钟燕,刘小虎,等. 扁塑藤素增强顺铂对条件性重编程原代肺癌细胞敏感性的机制[J]. 实用医学杂志,2022,38(7):841-847. doi: 10.3969/j.issn.1006-5725.2022.07.012 [20] 周鑫,杨进,陈林,等. 扁塑藤素对膀胱癌细胞增殖和凋亡的影响及其作用机制[J]. 中国癌症防治杂志,2023,15(1):18-24. [21] Condello M,Pellegrini E,Caraglia M,et al. Targeting autophagy to overcome human diseases[J]. Int J Mol Sci,2019,20(3):725. doi: 10.3390/ijms20030725 [22] Yun C W,Lee S H. The roles of autophagy in cancer[J]. Int J Mol Sci,2018,19(11):725. [23] Rakesh R,PriyaDharshini L C,Sakthivel K M,et al. Role and regulation of autophagy in cancer[J]. Biochim Biophys Acta Mol Basis Dis,2022,1868(7):166400. doi: 10.1016/j.bbadis.2022.166400 [24] Milkovic L,Cipak G A,Cindric M,et al. Short overview of ros as cell function regulators and their implications in therapy concepts[J]. Cells,2019,8(8):793. doi: 10.3390/cells8080793 [25] Nascimbeni A C,Codogno P,Morel E. Local detection of ptdIns3p at autophagosome biogenesis membrane platforms[J]. Autophagy,2017,13(9):1602-1612. doi: 10.1080/15548627.2017.1341465 [26] Ko J H,Yoon S O,Lee H J,et al. Rapamycin regulates macrophage activation by inhibiting nlrp3 inflammasome-p38 mapk-nfκb pathways in autophagy- and p62-dependent manners[J]. Oncotarget,2017,8(25):40817-40831. doi: 10.18632/oncotarget.17256 期刊类型引用(6)
1. 林永年,黄亚兰,邓金兰,邱燕燕,黄秋燕,刘江福,郭如意,高艺鹏. 泉州地区艾滋病患者机会性感染现状及预后Nomogram预测模型的构建. 中国感染与化疗杂志. 2024(01): 25-31 .  百度学术
百度学术2. 栾伶俐,于保平,李苏华. 艾滋病合并机会性感染患者血清内毒素、淀粉样蛋白、 CD4~+T细胞水平变化及其与病毒载量的关系. 医学理论与实践. 2024(04): 664-666 .  百度学术
百度学术3. 俞亚转,张丽媛,吴坤亮,杜永国,朱传龙. 获得性免疫缺陷综合征合并脓毒症患者外周血中T淋巴细胞亚群研究. 临床和实验医学杂志. 2024(08): 813-816 .  百度学术
百度学术4. 毛蕾,高果,樊彩萍. 拉米夫定、替诺福韦及依非韦伦抗病毒方案在艾滋病治疗中的应用. 河南医学高等专科学校学报. 2023(05): 509-512 .  百度学术
百度学术5. 周巧玉,莫金荣,廖炯,覃舒扬,李春红,韦珠梅,黄晓娟. 比克恩丙诺片与一线ART抗病毒治疗HIV/AIDS的效果评价. 临床和实验医学杂志. 2023(20): 2168-2171 .  百度学术
百度学术6. 杨雨,洪怡,杨莉. 替诺福韦、拉米夫定、依非韦伦联合治疗对艾滋病患者临床效果、免疫功能和安全性分析. 解放军医药杂志. 2022(06): 122-125 .  百度学术
百度学术其他类型引用(0)
-







 下载:
下载:




 下载:
下载: