Correlation and Diagnostic Performance of Inflammatory Cytokines in Relation to Bone Mineral Density and β-CTX in Postmenopausal Women with Type 2 Diabetes Mellitus
-
摘要:
目的 分析绝经后2型糖尿病(type 2 diabetes mellitus,T2DM)患者炎症因子与骨密度(bone mineral density,BMD)、I型胶原C末端交联肽(β-C-terminal telopeptide of type I collagen,β-CTX)之间的相关性,评估炎症因子在绝经后T2DM合并骨质疏松症(osteoporosis,OP)诊断中的效能。 方法 筛选了2023年10月1日至2024年8月31日期间在云南省第三人民医院内分泌科住院的538例绝经后T2DM患者,最终纳入181例患者作为研究对象。根据骨密度测定结果,将其分为骨量减少组(共86例,-2.5<T值<-1.0)和骨质疏松组(共95例,T值≤-2.5)。收集并记录了患者的人口学指标、炎症相关指标、骨代谢指标和其他临床指标。 结果 (1)比较两组间年龄、T2DM病程、体质指数(body mass index,BMI)、C反应蛋白(C-reactive protein,CRP)、白介素-6 (interleukin-6,IL-6)、中性粒细胞与淋巴细胞比值(neutrophil-to-lymphocyte ratio,NLR)、单核细胞与淋巴细胞比值(monocyte-to-lymphocyte ratio,MLR)、全身免疫炎症指数(systemic immune-inflammation index,SII)、β-CTX、甲状旁腺激素(parathyroid hormone,PTH)、各部位BMD值差异有统计学意义(P < 0.05);(2)Spearman相关性分析结果显示,CRP、IL-6、NLR、MLR、SII与骨密度呈负相关性(P < 0.05),在骨质疏松组中CRP、IL-6与β-CTX呈正相关(P < 0.05);(3)多因素二元Logistic回归分析显示,CRP、IL-6、SII、年龄、β-CTX是患者发生OP的独立危险因素(OR > 1,B > 0),而BMI是发生OP的独立保护因素(OR < 1,B < 0);(4)ROC曲线分析显示,CRP、IL-6、NLR、MLR、SII对绝经后T2DM合并OP均具有诊断效能,其中CRP、IL-6和SII联合检测模型的诊断效能最高。 结论 炎症因子与骨密度、β-CTX存在相关性,且对诊断绝经后T2DM患者合并OP有预测价值。 Abstract:Objective To analyze the correlation between inflammatory factors and bone mineral density (BMD) as well as β-C-terminal telopeptide of type I collagen (β-CTX) in postmenopausal patients with type 2 diabetes mellitus (T2DM), and to evaluate the diagnostic efficacy of the inflammatory factors in postmenopausal T2DM patients with osteoporosis (OP). Methods A total of 538 postmenopausal women with T2DM, hospitalized in the Department of Endocrinology at the Third People's Hospital of Yunnan Province from October 1, 2023 to August 31, 2024, were screened, and 181 were ultimately included in the study. Based on bone mineral density, they were divided into the osteopenia group (86 patients, -2.5<T<-1.0) and the osteoporosis group (95 patients, T≤-2.5). Demographic data, inflammation-related indicators, bone metabolism markers, and other clinical parameters were collected and recorded. Results (1) The differences were statistically significant in age, duration of T2DM, body mass index (BMI), C-reactive protein (CRP), interleukin-6 (IL-6), neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), systemic immune-inflammation index (SII), β-CTX, parathyroid hormone (PTH), and BMD at various sites between the two groups (P < 0.05); (2) Spearman correlation analysis results indicated that CRP, IL-6, NLR, MLR, and SII were negatively correlated with bone mineral density (P < 0.05), and in the osteoporosis group, CRP and IL-6 were positively correlated with β-CTX (P < 0.05); (3) Multifactorial binary logistic regression analysis showed that CRP, IL-6, SII, age, and β-CTX were independent risk factors for the occurrence of OP in both groups (OR > 1, B > 0), while BMI was an independent protective factor for the occurrence of OP (OR < 1, B < 0); (4) ROC curve analysis showed that CRP, IL-6, NLR, MLR, and SII all demonstrated diagnostic efficacy for postmenopausal T2DM patients with osteoporosis, among which the combined detection model of CRP, IL-6, and SII exhibited the highest diagnostic efficacy. Conclusion Inflammatory markers are correlated with bone mineral density and β-CTX levels, and may have predictive value for diagnosing osteoporosis in postmenopausal T2DM patients. -
表 1 两组患者一般临床数据对比 [M(P25,P75)/n(%)]
Table 1. Comparative analysis of general clinical data between patient groups [M(P25,P75)/n(%)]
项目 骨量减少组(n=86) 骨质疏松组(n=95) Z/χ2 P 年龄(岁) 66(56,71) 70(63,74) −3.445 <0.001* 绝经年龄(岁) 50(50,52) 50(49,52) −0.095 0.873 T2DM病程(年) 5.5(2,13.25) 10(4,19) −2.529 0.011* BMI(kg/m2) 24.46(23.01,26.36) 22.9(21.48,25.24) −3.071 0.002* HbA1c(%) 8.13(6.96,10.67) 8.45(7.03,10.06) −0.232 0.817 合并疾病情况 55(64) 64(67.4) 0.234 0.629 *P < 0.05。 表 2 两组患者炎症指标、骨代谢指标及骨密度值对比 [M(P25,P75)/($\bar x \pm s $)]
Table 2. Comparison of inflammatory markers,bone metabolism indices,and bone density values between patient groups[M(P25,P75)/($\bar x \pm s $)]
指标 骨量减少组(n=86) 骨质疏松组(n=95) Z/T P CRP (mg/L) 0.98(0.32,1.70) 2.01(1.09,3.43) −5.098 <0.001* IL-6(pg/mL) 1.80(1.39,3.03) 3.82(2.03,6.38) −5.908 <0.001* NLR 1.51(1.02,2.04) 1.78(1.36,2.53) −2.996 0.003* MLR 0.14(0.11,0.18) 0.16(0.13,0.21) −2.466 0.014* PLR 98.26(74.22,127.84) 104.30(81.16,144.90) −1.804 0.071 SII 302.70(217.72,440.01) 377.90(268.80,601.68) −3.034 0.002* β-CTX (ng/mL) 0.37(0.27,0.50) 0.59(0.46,0.73) −6.703 <0.001* 25(OH)VD(nmol/L) 69.20(57.14,88.95) 66.67(52.47,78.61) −1.572 0.116 PTH(pmol/L) 6.06(4.28,7.76) 7.23(5.32,9.29) −2.937 0.003* L1-L4 BMD(g/cm2) 1.040 ± 0.108 0.868 ± 0.127 9.891 <0.001* 左侧股骨颈BMD值(g/cm2) 0.827 ± 0.077 0.690 ± 0.092 10.852 <0.001* 右侧股骨颈BMD值(g/cm2) 0.823 ± 0.071 0.691 ± 0.090 11.018 <0.001* 左髋关节全部BMD值(g/cm2) 0.910 ± 0.079 0.759 ± 0.102 11.153 <0.001* 右髋关节全部BMD值(g/cm2) 0.914 ± 0.082 0.762 ± 0.103 10.988 <0.001* *P < 0.05。 表 3 两组患者炎症因子与骨密度、β-CTX的相关性
Table 3. Correlation between inflammatory factors,bone density,and β-CTX in patient groups
指标 骨密度分级(n=181)
(1:骨量减少;2:骨质疏松)β-CTX 骨量减少组(n=86) 骨质疏松组(n=95) r P r P r P CRP 0.380 <0.001* −0.032 0.767 0.222 0.031* IL-6 0.440 <0.001* −0.057 0.604 0.378 <0.001* NLR 0.223 0.003* −0.098 0.370 0.016 0.879 MLR 0.184 0.013* −0.144 0.186 0.050 0.632 PLR 0.134 0.071 0.102 0.351 −0.109 0.295 SII 0.226 0.002* −0.057 0.600 0.026 0.801 *P < 0.05。 表 4 两组患者骨质疏松影响因素的多因素二元Logistic回归分析
Table 4. Multivariate binary logistic regression of osteoporosis risk factors in two patient groups
因变量 自变量 B OR 95%CI P 是否发生
骨质疏松CRP 0.283 1.327 (1.005-1.754) 0.046* IL-6 0.302 1.352 (1.045-1.749) 0.022* NLR −0.462 0.630 (0.220-1.806) 0.390 MLR×100 −0.047 0.954 (0.867-1.050) 0.339 SII 0.004 1.004 (1.000-1.007) 0.035* 年龄 0.086 1.090 (1.024-1.160) 0.007* T2DM病程 0.040 1.041 (0.977-1.109) 0.220 BMI值 −0.209 0.811 (0.706-0.932) 0.003* β-CTX×100 0.056 1.058 (1.031-1.085) <0.001* PTH 0.118 1.125 (0.957-1.322) 0.153 * P < 0.05。 表 5 炎症因子对绝经后T2DM合并OP的诊断效能分析
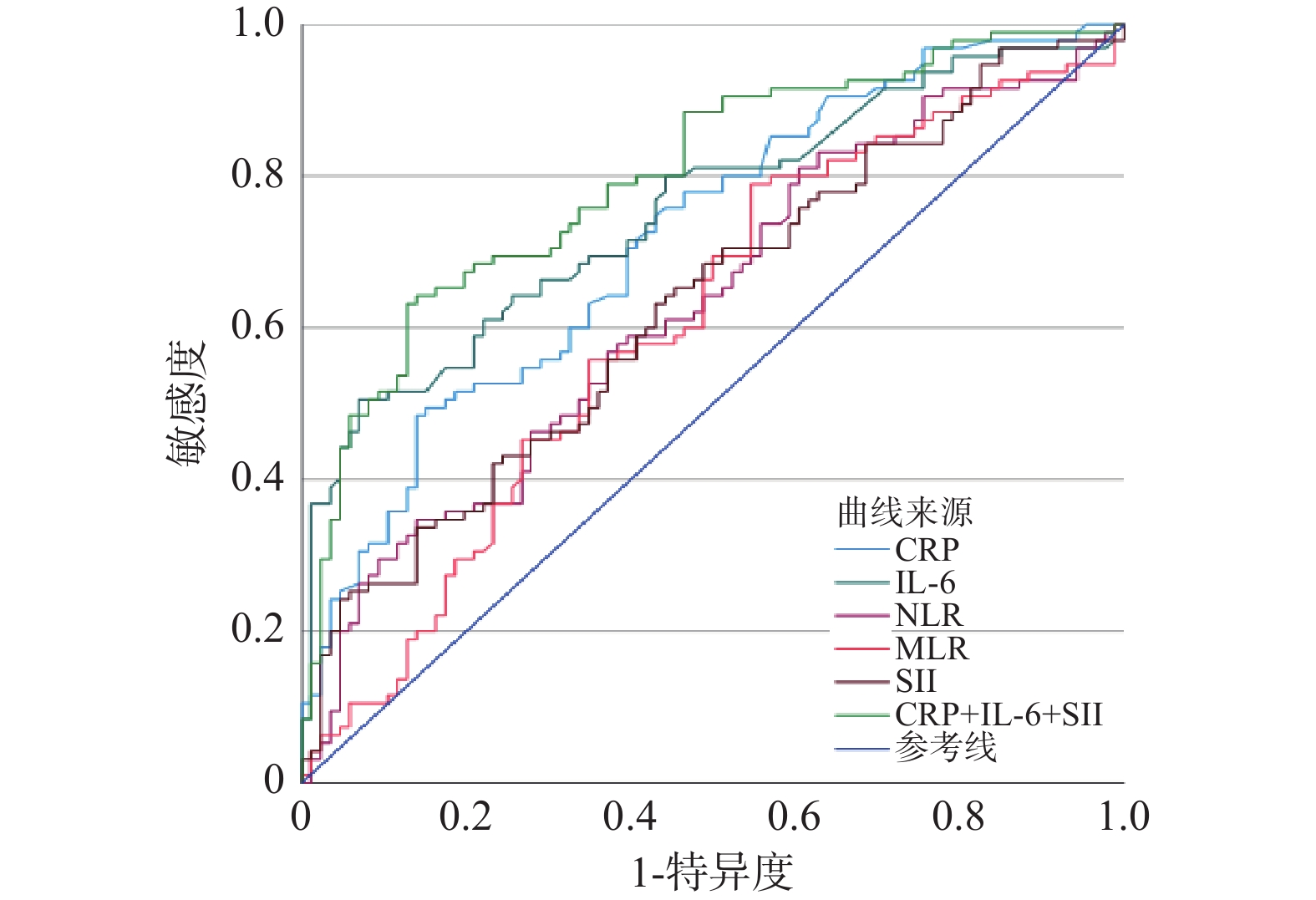
Table 5. Diagnostic efficacy of inflammatory factors for postmenopausal T2DM with osteoporosis
指标 AUC 约登指数 临界值 灵敏度 特异度 95%CI P CRP 0.720 0.344 2.060 0.495 0.849 (0.646-0.793) <0.001* IL-6 0.754 0.435 3.780 0.505 0.930 (0.684-0.824) <0.001* NLR 0.629 0.207 2.162 0.347 0.860 (0.548-0.710) 0.003* MLR 0.606 0.242 0.130 0.789 0.453 (0.523-0.689) 0.014* SII 0.631 0.202 325.851 0.632 0.570 (0.550-0.711) 0.002* CRP+IL-6+SII 0.799 0.504 0.569 0.632 0.872 (0.735-0.863) <0.001* *P < 0.05。 -
[1] Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus[J]. International Journal of Molecular Sciences, 2020, 21(17): 6275. doi: 10.3390/ijms21176275 [2] Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045[J]. Diabetes Research and Clinical Practice, 2022, 183(1): 109119. doi: 10.1016/j.diabres.2021.109119 [3] Li Y, Teng D I, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American diabetes association: National cross sectional study[J]. BMJ, 2020, 369(2): m997. [4] 中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2020年版)[J]. 中华糖尿病杂志, 2021, 13(4): 315-409. [5] Sharma P, Kumar R, Gaur K. Understanding the impact of diabetes on bone health: A clinical review[J]. Metabolism Open, 2024, 24(4): 100330. doi: 10.1016/j.metop.2024.100330 [6] Schacter G I, Leslie W D. Diabetes and osteoporosis: part I, epidemiology and pathophysiology[J]. Endocrinology and Metabolism Clinics, 2021, 50(2): 275-285. doi: 10.1016/j.ecl.2021.03.005 [7] Li J, Chen X, Lu L, et al. The relationship between bone marrow adipose tissue and bone metabolism in postmenopausal osteoporosis[J]. Cytokine & Growth Factor Reviews, 2020, 52(2): 88-98. [8] Cao Y, Dong B, Li Y, et al. Association of type 2 diabetes with osteoporosis and fracture risk: A systematic review and meta-analysis[J]. Medicine, 2025, 104(6): e41444. doi: 10.1097/MD.0000000000041444 [9] Soh G T, Mohammad A H, Syed Isa S N L, et al. Comparison of cytokine profile between postmenopausal women with and without osteoporosis a case-control study[J]. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders), 2023, 23(6): 811–817. [10] Terkawi M A, Matsumae G, Shimizu T, et al. Interplay between inflammation and pathological bone resorption: Insights into recent mechanisms and pathways in related diseases for future perspectives[J]. International Journal of Molecular Sciences, 2022, 23(3): 1786. doi: 10.3390/ijms23031786 [11] Damani J J, De Souza M J, Strock N C A, et al. Associations between inflammatory mediators and bone outcomes in postmenopausal women: A cross-sectional analysis of baseline data from the prune study[J]. Journal of Inflammation Research, 2023, 16(1): 639-663. doi: 10.2147/JIR.S397837 [12] Atlı H, Dayanan R. Evaluation of hematologic inflammatory markers in Graves’ disease[J]. Family Practice and Palliative Care, 2023, 8(5): 112-117. doi: 10.22391/fppc.1322984 [13] Jarmuzek P, Kozlowska K, Defort P, et al. Prognostic values of systemic inflammatory immunological markers in glioblastoma: A systematic review and meta-analysis[J]. Cancers, 2023, 15(13): 3339. doi: 10.3390/cancers15133339 [14] Meng L, Yang Y, Hu X, et al. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: A systematic review and meta-analysis[J]. Journal of Translational Medicine, 2023, 21(1): 79. doi: 10.1186/s12967-023-03924-y [15] 中华医学会骨质疏松和骨矿盐疾病分会. 原发性骨质疏松症诊疗指南(2022)[J]. 中华骨质疏松和骨矿盐疾病杂志, 2022, 15(6): 573-611. [16] Ambikairajah A, Walsh E, Cherbuin N. A review of menopause nomenclature[J]. Reproductive Health, 2022, 19(1): 29. doi: 10.1186/s12978-022-01336-7 [17] 中华医学会骨质疏松和骨矿盐疾病分会. 原发性骨质疏松症诊疗指南(2022)[J]. 中华骨质疏松和骨矿盐疾病杂志, 2022, 15(6): 573-611. doi: 10.3969/j.issn.1674-2591.2022.06.001 [18] 滕晓洁, 王振刚, 唐冠兰, 等. 内分泌疾病与继发性骨质疏松症相关性的研究进展[J]. 实用中医内科杂志, 2023, 37(6): 59-62. [19] Rose J. Autoimmune connective tissue diseases: Systemic lupus erythematosus and rheumatoid arthritis[J]. Immunology and Allergy Clinics, 2023, 43(3): 613-625. [20] 李丹. 感染性疾病与炎症风暴[C]//中华医学会呼吸病学2020年会暨第二十一次全国呼吸病学学术会议论文集. 北京: 中华医学会, 2020: 462–470. [21] 陈孝平, 张英泽, 兰平, 等. 外科学(第10版)[M]. 北京: 人民卫生出版社, 2024: 613 [22] Vestergaard P. Drugs causing bone loss[M]//Reginster J Y, Burlet N. Bone regulators and osteoporosis therapy[M]. London: Academic Press, 2020: 475−497. [23] Sobh M M, Abdalbary M, Elnagar S, et al. Secondary osteoporosis and metabolic bone diseases[J]. Journal of Clinical Medicine, 2022, 11(9): 2382. doi: 10.3390/jcm11092382 [24] Huang R, Chen Y, Tu M, et al. Monocyte to high-density lipoprotein and apolipoprotein A1 ratios are associated with bone homeostasis imbalance caused by chronic inflammation in postmenopausal women with type 2 diabetes mellitus[J]. Frontiers in Pharmacology, 2022, 13(1): 1062999. doi: 10.3389/fphar.2022.1062999 [25] Ono T, Hayashi M, Sasaki F, et al. RANKL biology: Bone metabolism, the immune system, and beyond[J]. Inflammation and Regeneration, 2020, 40(1): 1-16. doi: 10.1186/s41232-019-0110-4 [26] Martiniakova M, Biro R, Penzes N, et al. Links among obesity, type 2 diabetes mellitus, and osteoporosis: Bone as a target[J]. International Journal of Molecular Sciences, 2024, 25(9): 4827. doi: 10.3390/ijms25094827 [27] Liu Q, Wu W, Yang J, et al. A GP130‐targeting small molecule, LMT‐28, reduces LPS‐induced bone resorption around implants in diabetic models by inhibiting IL‐6/GP130/JAK2/STAT3 signaling[J]. Mediators of Inflammation, 2023, 2023(1): 9330439. [28] Xu J, Yu L, Liu F, et al. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review[J]. Frontiers in Immunology, 2023, 14(1): 1222129. doi: 10.3389/fimmu.2023.1222129 [29] Wang J, Chen J, Zhang B, et al. IL-6 regulates the bone metabolism and inflammatory microenvironment in aging mice by inhibiting Setd7[J]. Acta Histochemica, 2021, 123(5): 151718. doi: 10.1016/j.acthis.2021.151718 [30] Shahen V A, Gerbaix M, Koeppenkastrop S, et al. Multifactorial effects of hyperglycaemia, hyperinsulinemia and inflammation on bone remodelling in type 2 diabetes mellitus[J]. Cytokine & Growth Factor Reviews, 2020, 55(5): 109-118. [31] Mouliou D S. C-reactive protein: pathophysiology, diagnosis, false test results and a novel diagnostic algorithm for clinicians[J]. Diseases, 2023, 11(4): 132. doi: 10.3390/diseases11040132 [32] Ogbonna A, Ohotu E, Nwobodo E. The neutrophil to lymphocyte ratio: an emerging diagnostic biomarker for parainflammation[J]. International Blood Research & Reviews, 2022, 13(1): 130-133. [33] Takagi R, Sakamoto E, Kido J, et al. S100A9 increases IL-6 and RANKL Expressions through MAPKs and STAT3 signaling pathways in osteocyte-like cells[J]. BioMed Research International, 2020, 2020(1): 7149408. [34] Abid S, Lee M J, Rodich B, et al. Evaluation of an association between RANKL and OPG with bone disease in people with cystic fibrosis[J]. Journal of Cystic Fibrosis, 2023, 22(1): 140-145. doi: 10.1016/j.jcf.2022.08.011 [35] Dan J, Tan J, Huang J, et al. Early changes of platelet-lymphocyte ratio correlate with neoadjuvant chemotherapy response and predict pathological complete response in breast cancer[J]. Molecular and Clinical Oncology, 2023, 19(5): 90. doi: 10.3892/mco.2023.2686 [36] Shindo S, Savitri I J, Ishii T, et al. Dual-function semaphorin 4D released by platelets: Suppression of osteoblastogenesis and promotion of osteoclastogenesis[J]. International Journal of Molecular Sciences, 2022, 23(6): 2938. doi: 10.3390/ijms23062938 [37] Tang Y, Peng B, Liu J, et al. Systemic immune-inflammation index and bone mineral density in postmenopausal women: A cross-sectional study of the national health and nutrition examination survey (NHANES) 2007-2018[J]. Frontiers in Immunology, 2022, 13(1): 975400. doi: 10.3389/fimmu.2022.975400 [38] Fischer V, Haffner-Luntzer M. Interaction between bone and immune cells: implications for postmenopausal osteoporosis[J]. Seminars in Cell & Developmental Biology, 2022, 123(3): 14-21. -





 下载:
下载: