Inhibitory Effect of Crocin on Pituitary Adenomas via IRF7/NF-κB Signaling Pathway
-
摘要:
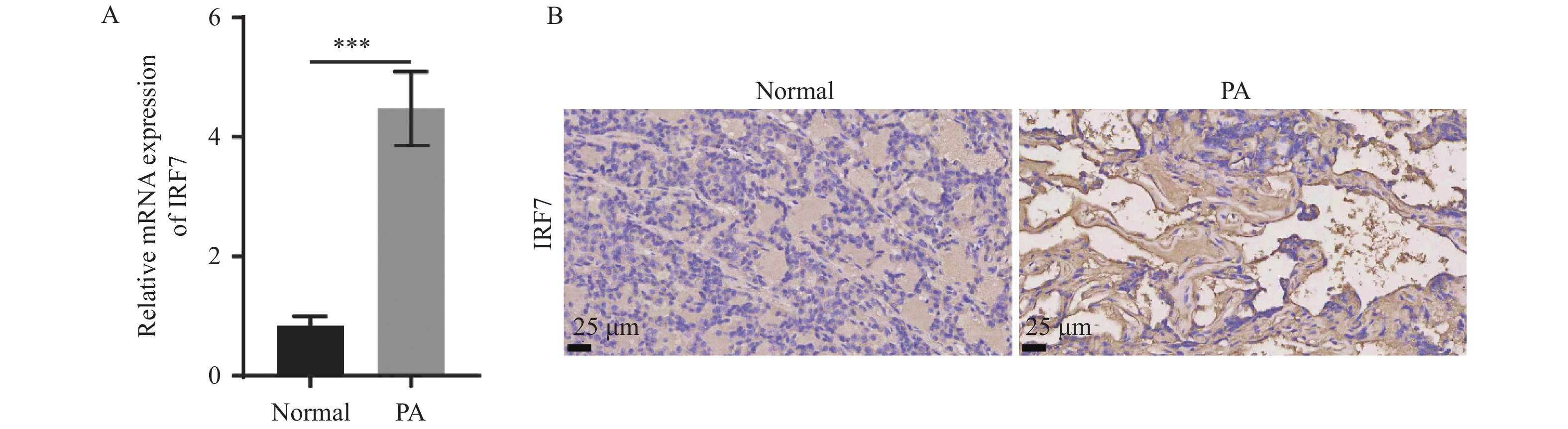
目的 通过临床样本及垂体腺瘤HP75细胞的相关分子生物学实验探讨藏红花素(Crocin)在垂体腺瘤(PA)中的作用及其机制。 方法 收集2022年6月至2023年5月昆明医科大学第一附属医院神经外二科及耳鼻咽喉颅底外科16例PA样本,3例正常对照垂体组织样本来自于昆明医科大学法医学院人体解剖。通过对临床样本检测IRF7 mRNA表达量,敲低HP75细胞IRF7表达检测增殖、迁移、侵袭及凋亡能力;进一步检测HP75细胞中IRF7调控NF-κB表达,以及藏红花素调控PA细胞的生长及其对IRF7/NF-κB信号通路调控作用。 结果 RT-qPCR检测及免疫组化显示,与正常对照组相比,PA中IRF7 mRNA的表达量增加(P < 0.001);si-IRF7组的IRF7蛋白表达量降低(P < 0.001);CCK-8、Transwell及流式细胞术检测结果显示,与对照组相比,敲低IRF7降低HP75细胞的细胞活力(P < 0.001),抑制HP75细胞的迁移和侵袭(P < 0.001),促进HP75细胞凋亡(P < 0.001)。此外,敲低IRF7能抑制p-NF-κB p65/NF-κB p65的表达(P < 0.001),抑制p-NF-κB p65/NF-κB p65的表达(P < 0.001);而过表达IRF7能部分逆转Crocin的作用(P < 0.001),p-NF-κB p65/NF-κB p65的表达(P < 0.01);最后,HP75细胞的生物学行为检测结果显示,与Crocin组相比,过表达IRF7能提高HP75细胞的细胞活力,同时促进其迁移和侵袭,抑制细胞凋亡(P < 0.001)。 结论 Crocin处理能抑制PA细胞的增殖、迁移和侵袭,并促进细胞凋亡,缓解PA的发展进程。在机制上,IRF7在PA中高表达,敲低IRF7能抑制PA的恶性生长;Crocin抑制PA细胞增殖、迁移、侵袭,促进细胞凋亡的作用可通过抑制IRF7/NF-κB信号通路实现。 Abstract:Objective To explore the role and mechanism of crocin in pituitary adenoma (PA) through clinical samples and related molecular biology experiments of HP75 cells. Methods From June 2022 to May 2023, 16 PA samples were collected from the Second Department of Neurology and Otolaryngology skull base surgery of the First Affiliated Hospital of Kunming Medical University. Three normal control samples were from the human anatomy of the Forensic College of Kunming Medical University. The expression of IRF7 mRNA in clinical samples was detected, and the proliferation, migration, invasion and apoptosis of HP75 cells were detected by knocking down the expression of IRF7; the expression of NF-κB was regulated by IRF7 in HP75 cells, and crocin regulated the growth of PA cells and its regulatory effect on IRF7/NF-κB signaling pathway. Results RT-qPCR and immunohisto-chemistry showed that compared with the normal control group, the expression of IRF7 mRNA in PA was significantly increased (P < 0.001); the expression of IRF7 protein in si-IRF7 group was significantly decreased (P < 0.001); CCK-8, Transwell and flow cytometry results showed that compared with the control group, knockdown of IRF7 significantly decreased the cell viability of HP75 cells (P < 0.001), inhibited the migration and invasion (P < 0.001), and promoted the apoptosis of HP75 cells (P < 0.001). In addition, knockdown of IRF7 could inhibit the expression of p-NF-κB p65/NF-κB p65 (P < 0.001) and p-NF-κB p65/NF-κB p65 (P < 0.001). Overexpression of IRF7 partially reversed the effect of crocin (P < 0.001) and restored the expression of p-NF-κB p65/NF-κB p65 (P < 0.01). Finally, the biological behavior of HP75 cells showed that compared with crocin group, overexpression of IRF7 could improve the cell viability of HP75 cells, promote their migration and invasion, and inhibit cell apoptosis (P < 0.001). Conclusion Crocin treatment can inhibit the proliferation, migration and invasion of PA cells, promote cell apoptosis, and alleviate the development of PA. In the mechanism, IRF7 is significantly overexpressed in PA, and knockdown of IRF7 can inhibit the malignant growth of PA. Crocin can inhibit the proliferation, migration and invasion of PA cells, and promote apoptosis by inhibiting IRF7/NF-κB signaling pathway. -
Key words:
- Pituitary adenoma /
- Crocetin /
- IRF7/NF -κB signaling pathway /
- Migration /
- Invasion /
- Apoptosis
-
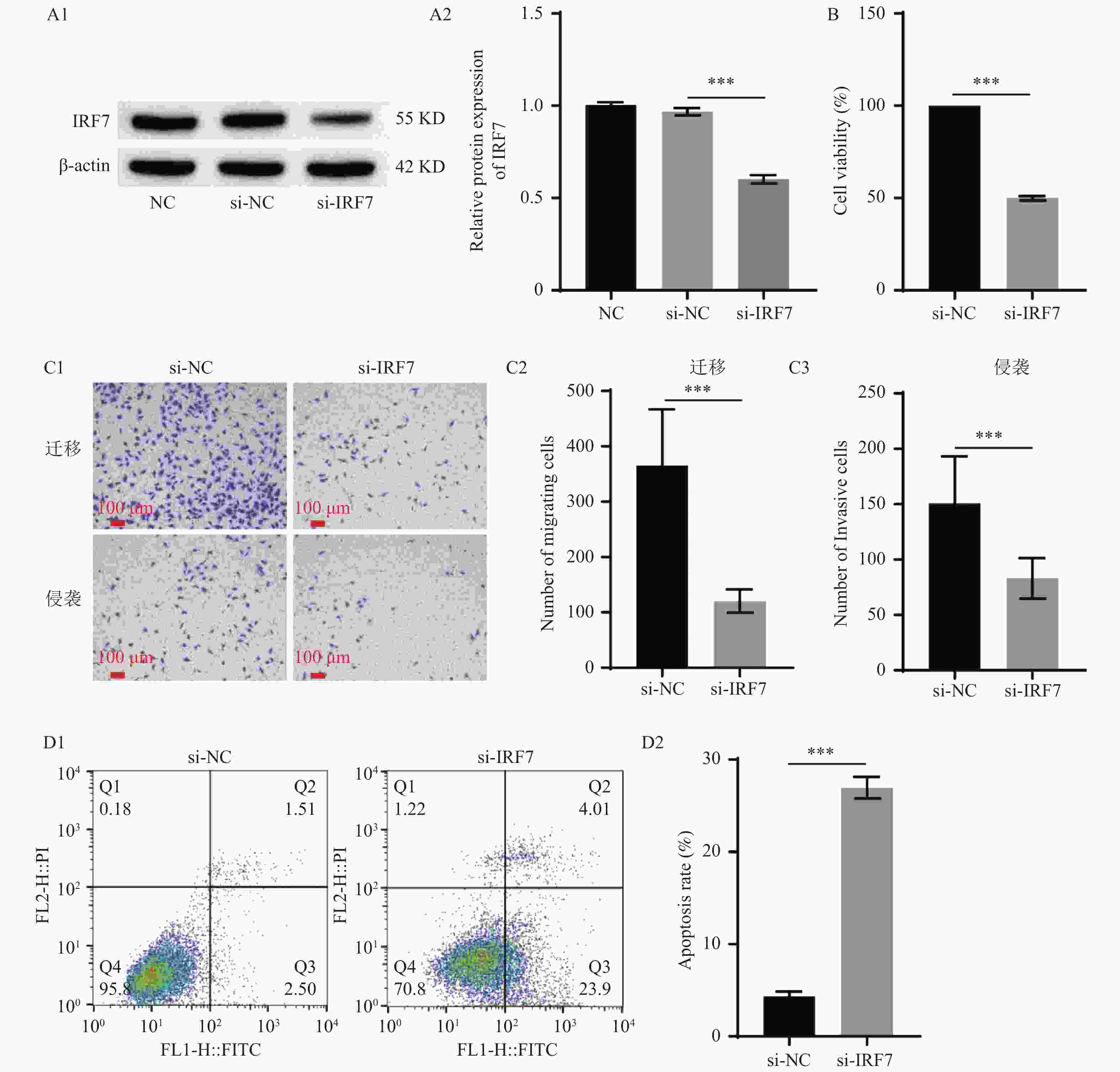
图 2 敲低IRF7抑制垂体腺瘤细胞增殖、迁移、侵袭,促进凋亡($\bar x \pm s $,n = 3)
A:Western blot检测IRF7的转染效率(A1:Western blot检测IRF7的蛋白表达电泳图,A2:IRF7蛋白表达的统计学分析);B:CCK-8检测HP75细胞的细胞活力;C:Transwell检测HP75细胞迁移和侵袭(100×)(C1:Transwell检测HP75细胞迁移和侵袭,C2:HP75细胞迁移统计学分析,C3:HP75细胞侵袭统计学分析);D:流式细胞术检测HP75细胞凋亡(D1:流式细胞术检测HP75细胞凋亡,D2:HP75细胞凋亡统计学分析)。***P < 0.001。
Figure 2. Knocking down IRF7 inhibited the proliferation, migration, and invasion of pituitary adenoma cells and promoted apoptosis($\bar x \pm s $,n = 3)
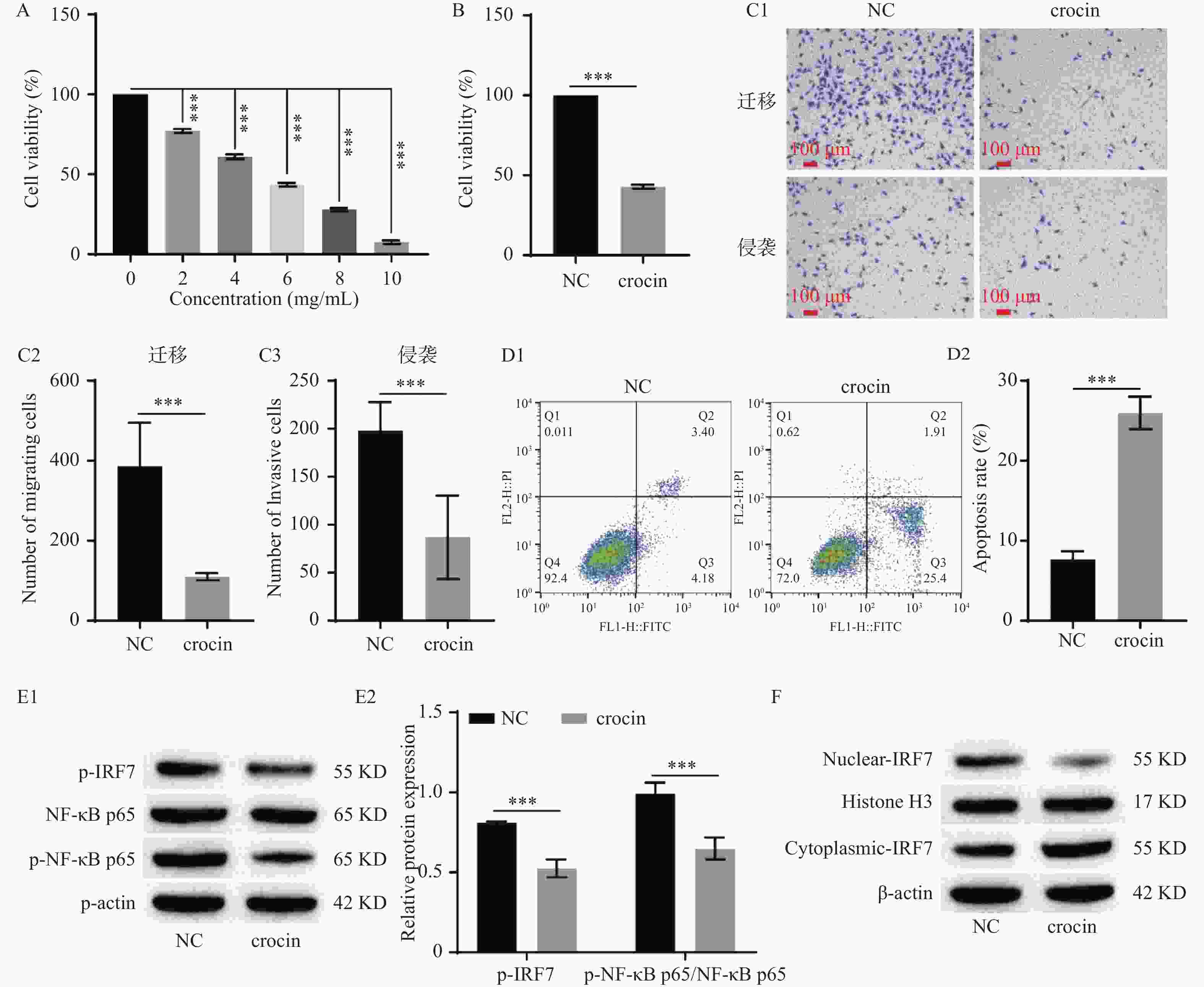
图 4 藏红花素抑制垂体腺瘤细胞恶性生长,且调控IRF7/NF-κB信号通路($\bar x \pm s $,n = 3)
A:Crocin浓度梯度处理后CCK-8检测HP75细胞的细胞活力;B:CCK-8检测HP75细胞的细胞活力;C:Transwell检测HP75细胞迁移和侵袭(100×)(C1:Transwell检测HP75细胞迁移和侵袭,C2:HP75细胞迁移统计学分析,C3:HP75细胞侵袭统计学分析);D:流式细胞术检测HP75细胞凋亡(D1:流式细胞术检测HP75细胞凋亡,D2:HP75细胞凋亡统计学分析);E:Western blot检测IRF7/NF-κB信号通路相关蛋白的表达水平(E1:Western blot检测IRF7/NF-κB信号通路相关蛋白表达的电泳图,E2:IRF7/NF-κB信号通路相关蛋白表达的统计学分析);F:Western blot检测IRF7的核转移。***P < 0.001。
Figure 4. Crocin inhibited the malignant growth of pituitary adenoma cells and regulated the IRF7/NF-κB signaling pathway($\bar x \pm s $,n = 3)
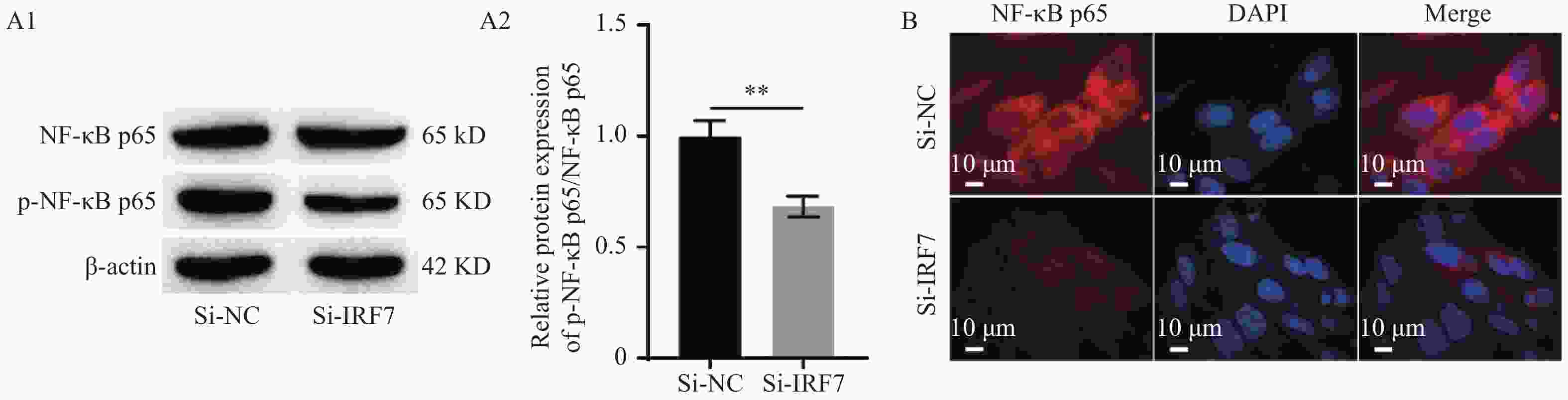
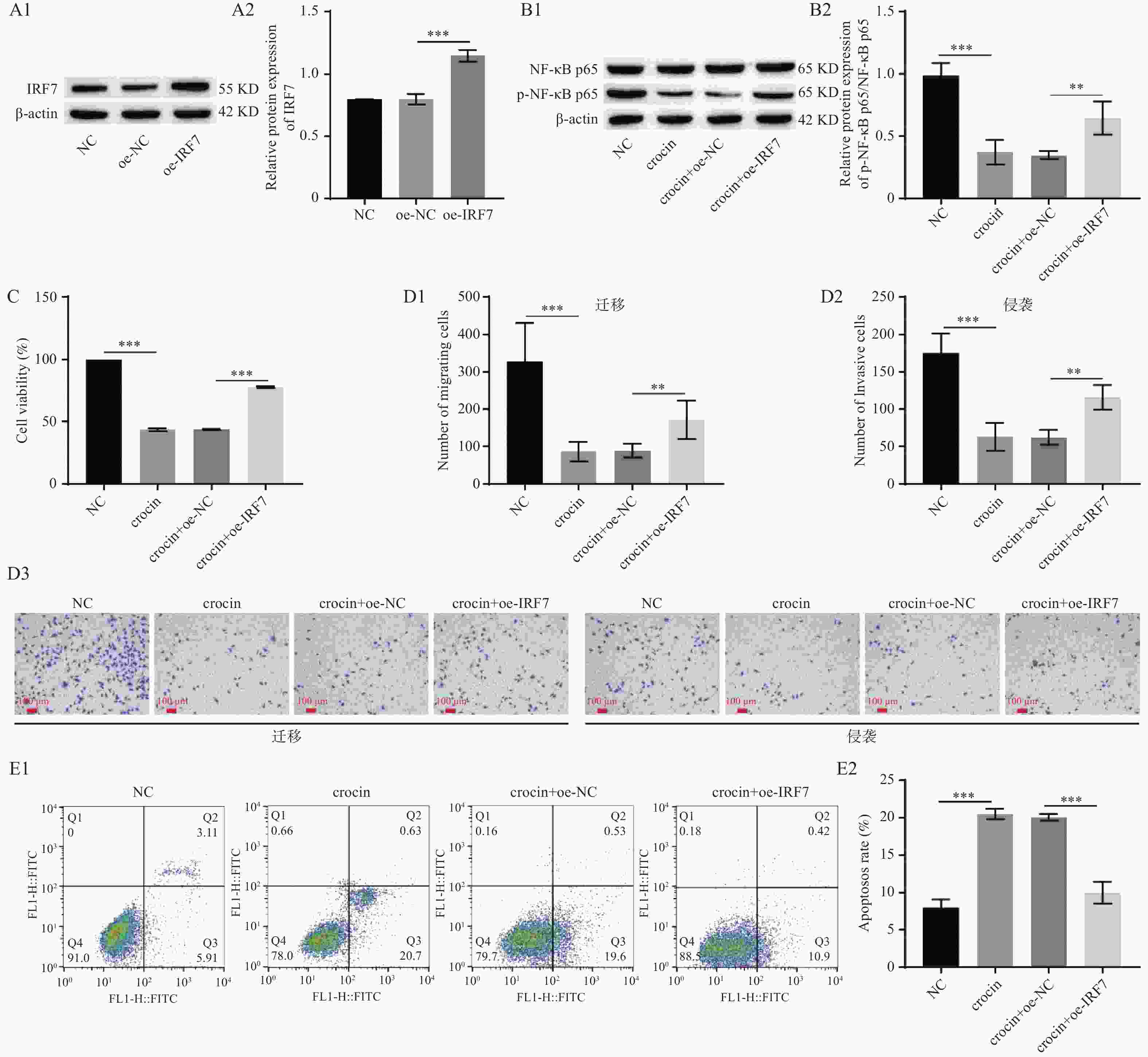
图 5 藏红花素抑制垂体腺瘤细胞恶性生长,调控IRF7/NF-κB信号通路($\bar x \pm s $,n = 3)
A:Western blot检测IRF7的过表达效率(A1:Western blot检测IRF7蛋白表达的电泳图,A2:IRF7蛋白表达的统计学分析);B:Western blot检测NF-κB p65和p-NF-κB p65的表达(B1:Western blot检测NF-κB p65和p-NF-κB p65蛋白表达的电泳图,B2:NF-κB p65和p-NF-κB p65蛋白表达的统计学分析);C:CCK-8检测HP75细胞的细胞活力;D:Transwell检测HP75细胞迁移和侵袭(100×)(D1:HP75细胞迁移统计学分析,D2:HP75细胞侵袭统计学分析,D3:Transwell检测HP75细胞迁移和侵袭);E:流式细胞术检测HP75细胞凋亡(E1:流式细胞术检测HP75细胞凋亡,E2:HP75细胞凋亡统计学分析)。**P < 0.01、***P < 0.001。
Figure 5. Crocin inhibited the malignant growth of pituitary adenoma cells and regulated the IRF7/NF-κB signaling pathway($\bar x \pm s $,n = 3)
表 1 引物序列
Table 1. Primer sequence
靶基因 序列(F:正向引物;R:反向引物)(5′-3′) IRF7 F:GCTGGACGTGACCATCATGTA R:GGGCCGTATAGGAACGTGC β-actin F:CATGTACGTTGCTATCCAGGC R:CTCCTTAATGTCACGCACGAT -
[1] Tritos NA, Miller KK. Diagnosis and management of pituitary adenomas: A review[J]. JAMA, 2023, 329(16): 1386-1398.Tritos NA,Miller KK. Diagnosis and management of pituitary adenomas: A review[J]. JAMA,2023,329(16):1386-1398. [2] Hlaváč m, Sommer F, Karpel-massler G, et al. Differential diagnosis and treatment of pituitary adenomas[J]. HNO,2019,67(4):307-318. [3] Jin L, Cai K, Wu W, et al. Correlations between the expression of molecules in the TGF-β signaling pathway and clinical factors in adamantinomatous craniopharyngiomas[J]. Front Endocrinol (Lausanne), 2023, 14(3): e1167776.Jin L,Cai K,Wu W,et al. Correlations between the expression of molecules in the TGF-β signaling pathway and clinical factors in adamantinomatous craniopharyngiomas[J]. Front Endocrinol (Lausanne),2023,14(3):e1167776. [4] Beylerli O, Gareev I, Pavlov V, et al. The role of long noncoding rnas in the biology of pituitary adenomas[J]. World Neurosurg, 2020, 137(5): 252-256.Beylerli O,Gareev I,Pavlov V,et al. The role of long noncoding rnas in the biology of pituitary adenomas[J]. World Neurosurg,2020,137(5):252-256. [5] Bukhari SI, Manzoor M, Dhar M K. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids[J]. Biomed Pharmacother, 2018, 98(2): 733-745.Bukhari SI,Manzoor M,Dhar M K. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids[J]. Biomed Pharmacother,2018,98(2):733-745. [6] Luo Y, Yu P, Zhao J, et al. Pathogenesis and anti-proliferation mechanisms of Crocin in human gastric carcinoma cells[J]. International Journal of Clinical and Experimental Pathology, 2020, 13(5): 912-922.Luo Y,Yu P,Zhao J,et al. Pathogenesis and anti-proliferation mechanisms of Crocin in human gastric carcinoma cells[J]. International Journal of Clinical and Experimental Pathology,2020,13(5):912-922. [7] Faraji S, Moosavi SA, Neshasteh-riz A, et al. Radioprotective effect of resveratrol, crocin, and their combination on cytogenetic alterations in human lymphocytes[J]. J Biomed Phys Eng, 2024, 14(3): 255-266.Faraji S,Moosavi SA,Neshasteh-riz A,et al. Radioprotective effect of resveratrol,crocin,and their combination on cytogenetic alterations in human lymphocytes[J]. J Biomed Phys Eng,2024,14(3):255-266. [8] Wang G, Zhang B, Wang Y, et al. Crocin promotes apoptosis of human skin cancer cells by inhibiting the JAK/STAT pathway[J]. Experimental and Therapeutic Medicine, 2018, 16(6): 5079-5084.Wang G,Zhang B,Wang Y,et al. Crocin promotes apoptosis of human skin cancer cells by inhibiting the JAK/STAT pathway[J]. Experimental and Therapeutic Medicine,2018,16(6):5079-5084. [9] Jia Y, Yang H, Yu J, et al. Crocin suppresses breast cancer cell proliferation by down-regulating tumor promoter miR-122-5p and up-regulating tumor suppressors FOXP2 and SPRY2[J]. Environmental toxicology, 2023, 38(7): 1597-1608.Jia Y,Yang H,Yu J,et al. Crocin suppresses breast cancer cell proliferation by down-regulating tumor promoter miR-122-5p and up-regulating tumor suppressors FOXP2 and SPRY2[J]. Environmental toxicology,2023,38(7):1597-1608. [10] Xiao Q, Li X, Li Y, et al. Biological drug and drug delivery-mediated immunotherapy[J]. Acta Pharm Sin B, 2021, 11(4): 941-960.Xiao Q,Li X,Li Y,et al. Biological drug and drug delivery-mediated immunotherapy[J]. Acta Pharm Sin B,2021,11(4):941-960. [11] Antonczyk A, Krist B, Sajek M, et al. Direct Inhibition of IRF-dependent transcriptional regulatory mechanisms associated with disease[J]. Frontiers in Immunology, 2019, 10(5): e01176.Antonczyk A,Krist B,Sajek M,et al. Direct Inhibition of IRF-dependent transcriptional regulatory mechanisms associated with disease[J]. Frontiers in Immunology,2019,10(5):e01176. [12] Li W, Wang Q, Feng Q, et al. Oncogenic KSHV-encoded interferon regulatory factor upregulates HMGB2 and CMPK1 expression to promote cell invasion by disrupting a complex lncRNA-OIP5-AS1/miR-218-5p network[J]. PLoS Pathogens, 2019, 15(1): e1007578.Li W,Wang Q,Feng Q,et al. Oncogenic KSHV-encoded interferon regulatory factor upregulates HMGB2 and CMPK1 expression to promote cell invasion by disrupting a complex lncRNA-OIP5-AS1/miR-218-5p network[J]. PLoS Pathogens,2019,15(1):e1007578. [13] Li W, Wang F, Shi J, et al. Sperm associated antigen 9 promotes oncogenic KSHV-encoded interferon regulatory factor-induced cellular transformation and angiogenesis by activating the JNK/VEGFA pathway[J]. PLoS Pathogens, 2020, 16(8): e1008730.Li W,Wang F,Shi J,et al. Sperm associated antigen 9 promotes oncogenic KSHV-encoded interferon regulatory factor-induced cellular transformation and angiogenesis by activating the JNK/VEGFA pathway[J]. PLoS Pathogens,2020,16(8):e1008730. [14] Liao W, Overman M J, Boutin A T, et al. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer[J]. Cancer Cell, 2019, 35(4): 559-572.Liao W,Overman M J,Boutin A T,et al. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer[J]. Cancer Cell,2019,35(4):559-572. [15] Kazzaz S A, Tawil J, Harhaj E W. The aryl hydrocarbon receptor-interacting protein in cancer and immunity: Beyond a chaperone protein for the dioxin receptor[J]. The Journal of Biological Chemistry, 2024, 300(4): e107157.Kazzaz S A,Tawil J,Harhaj E W. The aryl hydrocarbon receptor-interacting protein in cancer and immunity: Beyond a chaperone protein for the dioxin receptor[J]. The Journal of Biological Chemistry,2024,300(4):e107157. [16] Zhang R, Yang F, Fan H, et al. Long non-coding RNA TUG1/microRNA-187-3p/TESC axis modulates progression of pituitary adenoma via regulating the NF-κB signaling pathway[J]. Cell Death & Disease, 2021, 12(6): e524.Zhang R,Yang F,Fan H,et al. Long non-coding RNA TUG1/microRNA-187-3p/TESC axis modulates progression of pituitary adenoma via regulating the NF-κB signaling pathway[J]. Cell Death & Disease,2021,12(6):e524. [17] Fan S, Popli S, Chakravarty S, et al. Non-transcriptional IRF7 interacts with NF-κB to inhibit viral inflammation[J]. The Journal of Biological Chemistry, 2024, 300(4): e107200.Fan S,Popli S,Chakravarty S,et al. Non-transcriptional IRF7 interacts with NF-κB to inhibit viral inflammation[J]. The Journal of Biological Chemistry,2024,300(4):e107200. [18] Xu Q, Yu J, Jia G, et al. Crocin attenuates NF-κB-mediated inflammation and proliferation in breast cancer cells by down-regulating PRKCQ[J]. Cytokine, 2022, 154(6): e155888.Xu Q,Yu J,Jia G,et al. Crocin attenuates NF-κB-mediated inflammation and proliferation in breast cancer cells by down-regulating PRKCQ[J]. Cytokine,2022,154(6):e155888. [19] Daly AF, Beckers A. The epidemiology of pituitary adenomas[J]. Endocrinol Metab Clin North Am, 2020, 49(3): 347-355.Daly AF,Beckers A. The epidemiology of pituitary adenomas[J]. Endocrinol Metab Clin North Am,2020,49(3):347-355. [20] Melmed S, Kaiser UB, Lopes MB, et al. Clinical biology of the pituitary adenoma[J]. Endocr Rev, 2022, 43(6): 1003-1037.Melmed S,Kaiser UB,Lopes MB,et al. Clinical biology of the pituitary adenoma[J]. Endocr Rev,2022,43(6):1003-1037. [21] Xi X, Liu N, Wang Q, et al. ACT001, a novel PAI-1 inhibitor, exerts synergistic effects in combination with cisplatin by inhibiting PI3K/AKT pathway in glioma[J]. Cell Death & Disease, 2019, 10(10): e757.Xi X,Liu N,Wang Q,et al. ACT001,a novel PAI-1 inhibitor,exerts synergistic effects in combination with cisplatin by inhibiting PI3K/AKT pathway in glioma[J]. Cell Death & Disease,2019,10(10):e757. [22] Salomon M P, Wang X, Marzese D M, et al. The epigenomic landscape of pituitary adenomas reveals specific alterations and differentiates among acromegaly, cushing's disease and endocrine-inactive subtypes[J]. Clinical cancer research: an official journal of the American Association for Cancer Research, 2018, 24(17): 4126-4136.Salomon M P,Wang X,Marzese D M,et al. The epigenomic landscape of pituitary adenomas reveals specific alterations and differentiates among acromegaly,cushing's disease and endocrine-inactive subtypes[J]. Clinical cancer research: an official journal of the American Association for Cancer Research,2018,24(17):4126-4136. [23] Zhong W, Yang W, Qin Y, et al. 6-Gingerol stabilized the p-VEGFR2/VE- cadherin/β-catenin/actin complex promotes microvessel normalization and suppresses tumor progression[J]. Journal of Experimental & Clinical Cancer Research: CR, 2019, 38(1): e285.Zhong W,Yang W,Qin Y,et al. 6-Gingerol stabilized the p-VEGFR2/VE- cadherin/β-catenin/actin complex promotes microvessel normalization and suppresses tumor progression[J]. Journal of Experimental & Clinical Cancer Research: CR,2019,38(1):e285. [24] Veisi A, AkbarI G, Mard S A, et al. Role of crocin in several cancer cell lines: An updated review[J]. Iranian Journal of Basic Medical Sciences, 2020, 23(1): 3-12.Veisi A,AkbarI G,Mard S A,et al. Role of crocin in several cancer cell lines: An updated review[J]. Iranian Journal of Basic Medical Sciences,2020,23(1):3-12. [25] Bakshi H A, Quinn G A, Nasef M M, et al. Crocin inhibits angiogenesis and metastasis in colon cancer via TNF-α/NF-κB/VEGF pathways[J]. Cells., 2022, 11(9): e1502.Bakshi H A,Quinn G A,Nasef M M,et al. Crocin inhibits angiogenesis and metastasis in colon cancer via TNF-α/NF-κB/VEGF pathways[J]. Cells.,2022,11(9):e1502. [26] Bi X, Jiang Z, Luan Z, et al. Crocin exerts anti-proliferative and apoptotic effects on cutaneous squamous cell carcinoma via miR-320a/ATG2B[J]. Bioengineered, 2021, 12(1): 4569-4580.Bi X,Jiang Z,Luan Z,et al. Crocin exerts anti-proliferative and apoptotic effects on cutaneous squamous cell carcinoma via miR-320a/ATG2B[J]. Bioengineered,2021,12(1):4569-4580. [27] Ma W, Huang G, Wang Z, et al. IRF7: role and regulation in immunity and autoimmunity[J]. Front Immunol, 2023, 14(10): e1236923.Ma W,Huang G,Wang Z,et al. IRF7: role and regulation in immunity and autoimmunity[J]. Front Immunol,2023,14(10):e1236923. [28] Lv Y, Sun S, Zhang J, et al. Loss of RBM45 inhibits breast cancer progression by reducing the SUMOylation of IRF7 to promote IFNB1 transcription[J]. Cancer Lett, 2024, 596(1): e216988.Lv Y,Sun S,Zhang J,et al. Loss of RBM45 inhibits breast cancer progression by reducing the SUMOylation of IRF7 to promote IFNB1 transcription[J]. Cancer Lett,2024,596(1):e216988. [29] Lin L, Cai J. Circular RNA circ-EGLN3 promotes renal cell carcinoma proliferation and aggressiveness via miR-1299-mediated IRF7 activation[J]. Journal of Cellular Biochemistry, 2020, 121(11): 4377-4385.Lin L,Cai J. Circular RNA circ-EGLN3 promotes renal cell carcinoma proliferation and aggressiveness via miR-1299-mediated IRF7 activation[J]. Journal of Cellular Biochemistry,2020,121(11):4377-4385. [30] Teng S, Hao J, Bi H, et al. The protection of crocin against ulcerative colitis and colorectal cancer via suppression of NF-κB-mediated inflammation[J]. Frontiers in Pharmacology, 2021, 12(3): e639458.Teng S,Hao J,Bi H,et al. The protection of crocin against ulcerative colitis and colorectal cancer via suppression of NF-κB-mediated inflammation[J]. Frontiers in Pharmacology,2021,12(3):e639458. -





 下载:
下载: