Effects of Dexmedetomidine on Inflammatory Response in Rats with Smoke Inhalation Lung Injury
-
摘要:
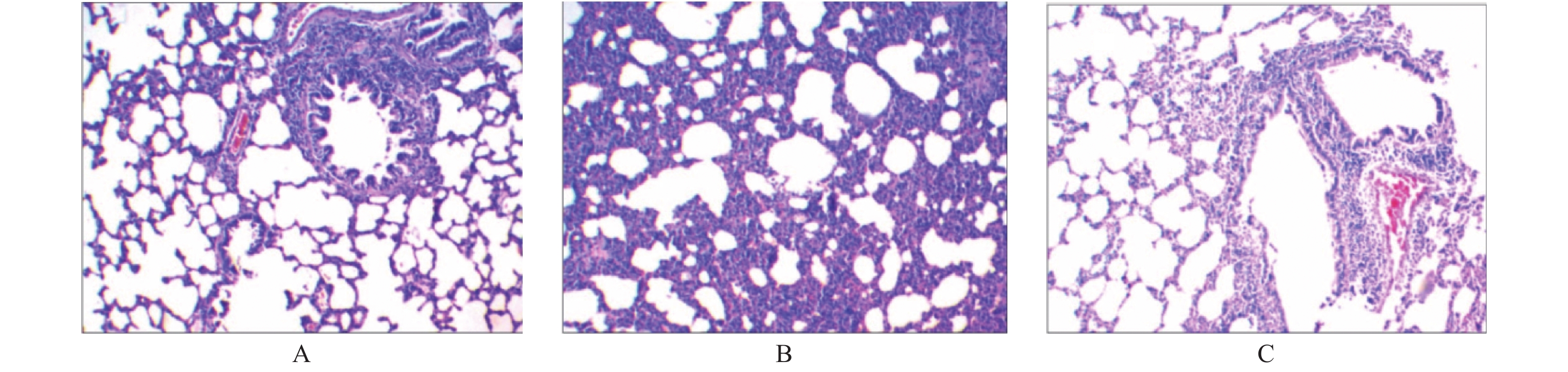
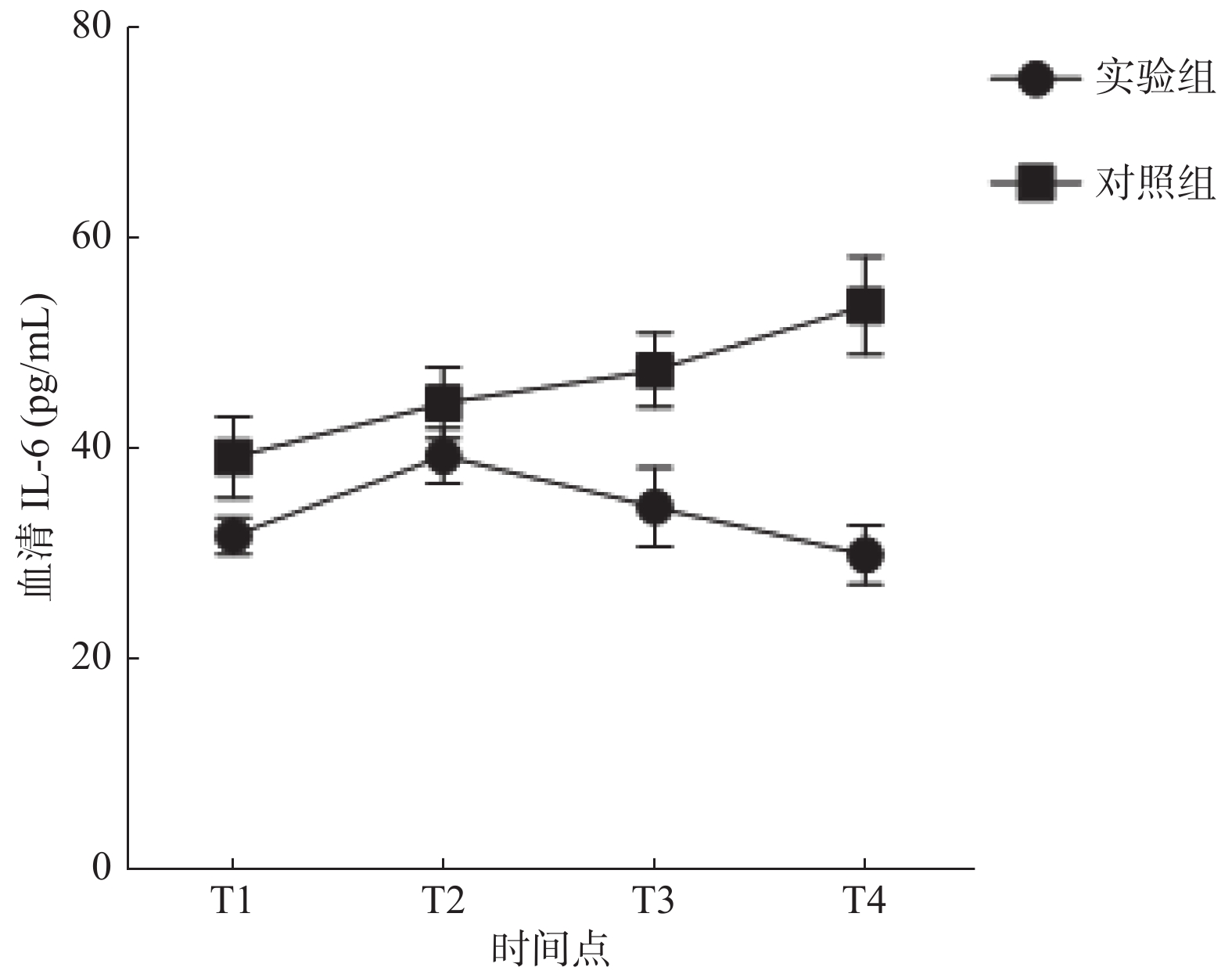
目的 探究右美托咪定(dexmedetomidine,DEX)对吸入性肺损伤大鼠肺组织炎症反应及炎性因子表达的影响。 方法 选择同批次饲养、清洁健康的成年SD大鼠70只,随机置于烟雾发生室内进行致伤(6只致伤后死亡),分为实验组(DEX 4.5 μg/kg静注,n = 32)、对照组(等量生理盐水静注,n = 32);余8只大鼠作为空白组,不予任何干预措施,正常饲养,用于观察动物模型及肺损伤情况。据给药时间点不同,将实验组及对照组大鼠分别均分为4个亚组,每组8只,分别于烟雾致伤成功后半小时(T1)、12 h(T2)、24 h(T3)、48 h(T4)4个时间点经尾静脉注射DEX溶液 或等容量生理盐水;于给药半小时后解剖采血,用酶联免疫吸附法(ELISA)测定大鼠血清、支气管肺泡灌洗液肿瘤坏死因子 α(TNF-α)、白细胞介素 6(IL-6)水平,留取肺组织标本观察大鼠致伤后各时点支气管、肺泡光镜下病理变化,与空白组对比。 结果 与空白组健康大鼠比较,所有致伤大鼠均建模成功。对照组大鼠血清及肺泡灌洗液中的炎症因子(TNF-α、IL-6)浓度较空白组、实验组升高,三组比较差异具有统计学意义(P < 0.05)。与对照组比较,实验组各时间点血清、肺泡灌洗液中TNF-α、IL-6水平均降低( P < 0.05),尤以(T4)时最为明显。血清中,对照组TNF-α、IL-6均随时间增加呈持续上升趋势( P < 0.05),实验组TNF-α、IL-6均呈先升高后下降趋势,于12 h达高峰,随后缓慢下降( P < 0.05);肺泡灌洗液中,实验组炎症因子随着时间增加呈先上升后下降趋势,其中IL-6于12 h达峰后下降,TNF-α于24 h达峰后下降( P < 0.05)。相比空白组,光镜下实验组、对照组大鼠肺泡周围均可见大量炎性细胞浸润;对照组以中-重度炎症反应为主,实验组以轻度炎症反应为主( P < 0.05)。 结论 右美托咪定可抑制吸入性肺损伤大鼠体内炎性因子表达,减轻肺组织炎症反应,从而起到肺保护作用。 Abstract:Objective To investigate the effects of dexmedetomidine (DEX) on inflammatory response and expression of inflammatory factors in lung tissue of rats with smoke inspiratory lung injury. Methods Seventy-eight healthy and clean adult SD rats were selected from the same batch. Among them, the rats were randomly selected to be injured in the smoke chamber(six rats died)and divided into experimental group (DEX 4.5 ug/kg static injection, n = 32) and control group (same amount of normal saline static injection, n = 32). The remaining 8 rats were used as the blank group without any intervention.According to the different time points of administration, the experimental group and the control group were divided into 4 subgroups, with 8 in each group. The DEX solution was injected through the tail-vein at 4 time points after the smoke injury: half an hour (T1), 12 hours (T2), 24 hours (T3) and 48 hours (T4), respectively.Blood samples were dissected and collected half an hour after administration, and serum and bronchoalveolar lavage fluid levels of tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) were measured by ELISA. Lung tissue was taken to observe the pathological changes of bronchi and alveoli at each time point after injury, and the results were compared with those of the healthy group. Results Compared with the healthy rats in the blank group, all the injured rats were successfully modeled.Compared with the control group, the serum and alveolar lavage fluid of the experimental group showed reduced levels of TNF-αand IL-6 (P < 0.05), especially in the T4stage. TNF-αand IL-6 in the serum of the control group increased continuously with time ( P < 0.05), while TNF-αand IL-6 in the experimental group first increased and then decreased, reaching a peak at 12 hours, and then decreased slowly ( P < 0.05). In alveolar lavage fluid, the inflammatory cytokines in the experimental group increased first and then decreased with time, in which il-6 decreased after reaching the peak at 12 hours, and TNF- radiation decreased after reaching the peak at 24 hours ( P < 0.05). Compared with the healthy group, a large number of inflammatory cells were observed around the alveoli of rats in the experimental group and control group under the light microscope. Moderate and severe inflammatory reactions were dominant in the control group, and mild inflammatory reactions were dominant in the experimental group ( P < 0.05). Conclusion Dexmedetomidine can significantly down-regulate the expression of inflammatory factors in rats with inhalation lung injury, promote the repair of lung tissue injury, and thus play a protective role in lung. -
Key words:
- Dexmedetomidine /
- Inhalation lung injury /
- Burns /
- Inflammatory cytokines /
- Rats
-
表 1 大鼠血清及肺泡灌洗液炎性因子(IL-6、TNF-α)的比较[( $\bar x \pm s$),pg/mL]
Table 1. Compared with inflammatory factors in serum and alveolar lavage fluid of rats[( $\bar x \pm s$),pg/mL]
组别 血清 肺泡灌洗液 TNF-α IL-6 TNF-α IL-6 实验组 284.89 ± 9.39 31.68 ± 1.73 284.89 ± 9.39 42.01 ± 8.14 对照组 310.21 ± 13.94* 39.16 ± 3.88* 310.21 ± 13.95* 50.09 ± 5.65* 空白组 245.34 ± 9.59* 23.34 ± 2.66* 245.34 ± 9.59* 33.09 ± 3.49* 与实验组比较,*P < 0.05。 表 2 大鼠血清炎性因子变化水平( $\bar x \pm s$)
Table 2. Change level of inflammatory factor in serum of rats( $\bar x \pm s$)
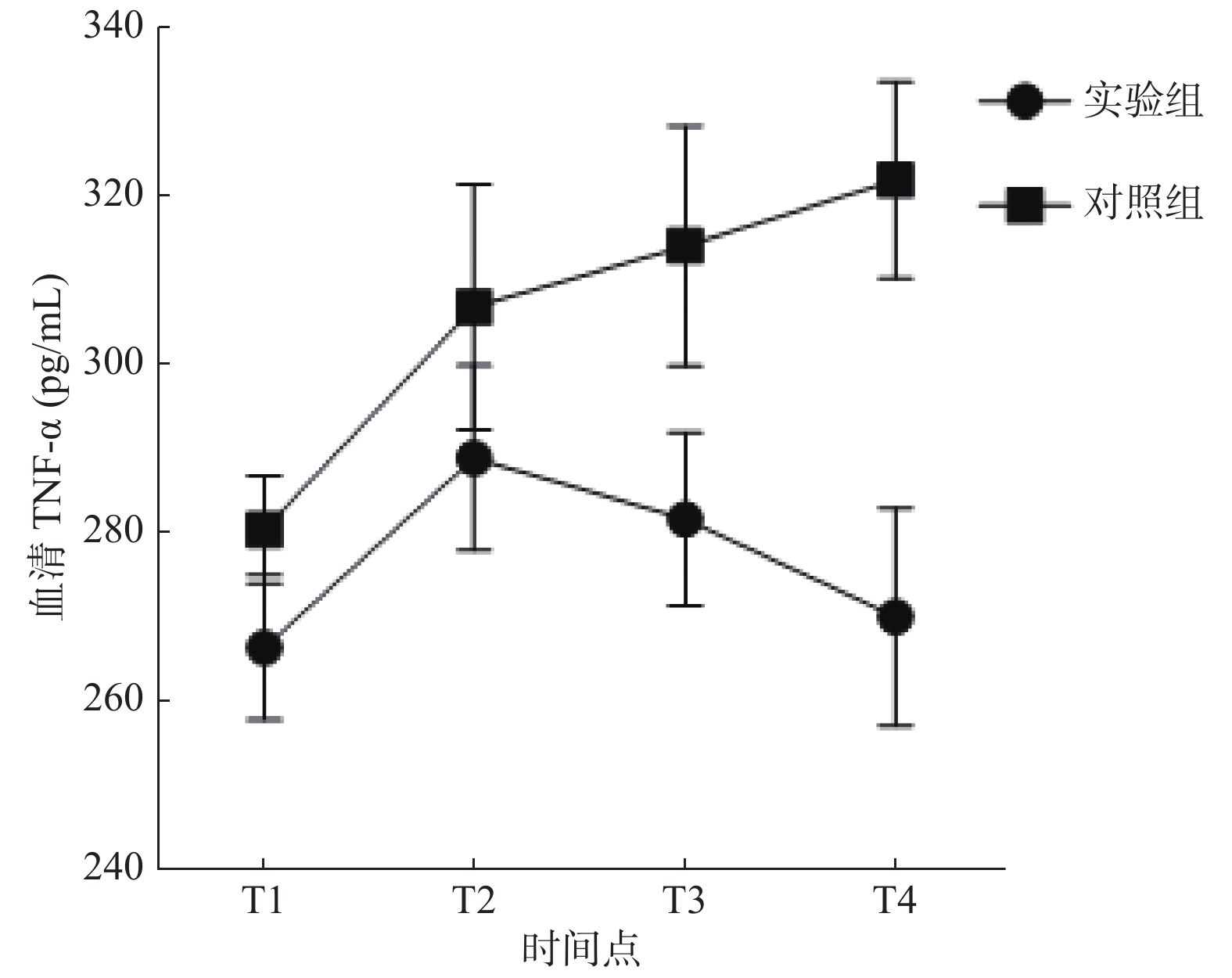
炎性因子 组别 T1 T2 T3 T4 IL-6(pg/mL) 实验组 31.68 ± 1.74 39.28 ± 2.61 34.43 ± 3.76 29.90 ± 2.78 对照组 39.16 ± 3.88* 44.30 ± 3.43* 47.44 ± 3.53* 53.54 ± 4.67* TNF-α(pg/mL) 实验组 266.32 ± 8.59 288.79 ± 10.99 281.52 ± 10.26 269.92 ± 12.98 对照组 280.32 ± 6.41* 306.68 ± 14.63* 313.96 ± 14.29* 321.82 ± 11.72* 与实验组比较,*P < 0.05。 表 3 大鼠肺泡灌洗液炎性因子变化水平( $\bar x \pm s$)
Table 3. Change level of inflammatory factor in alveolar lavage fluid of rats( $\bar x \pm s$)
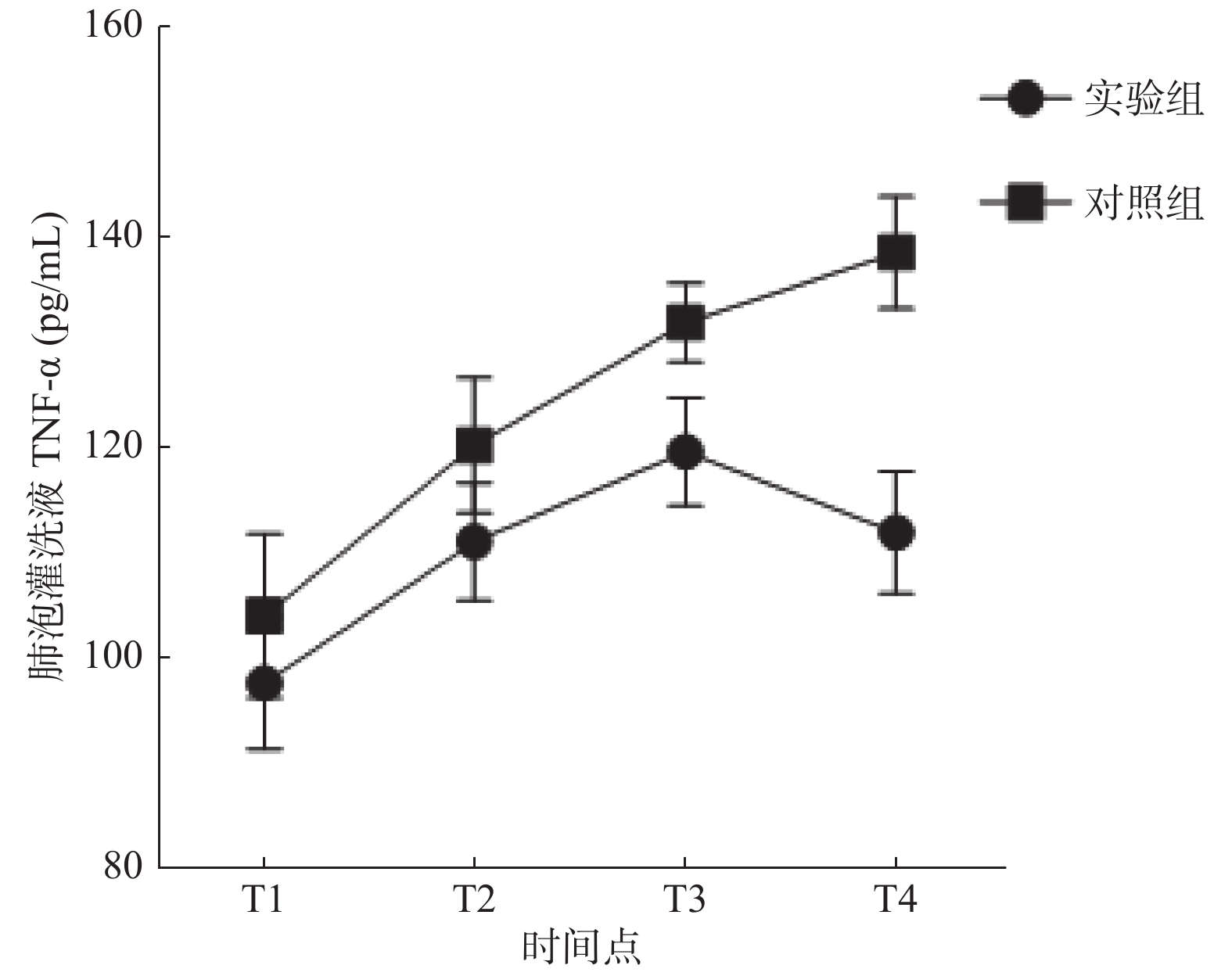
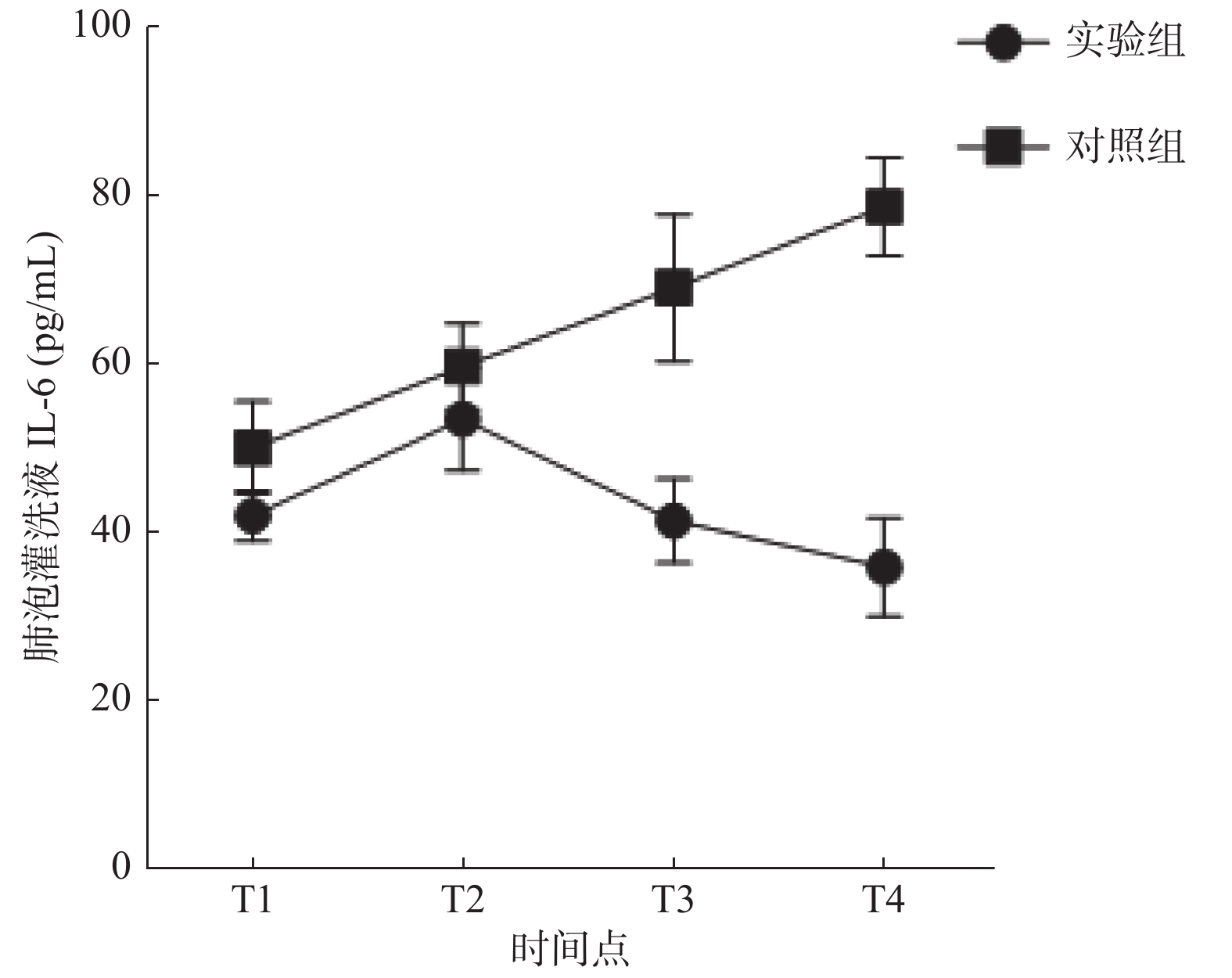
炎性因子 组别 T1 T2 T3 T4 IL-6(pg/mL) 实验组 42.01 ± 3.02 53.53 ± 6.15 41.44 ± 4.99 35.99 ± 5.89 对照组 50.09 ± 5.65* 59.81 ± 5.09* 69.10 ± 8.80* 78.78 ± 5.82* TNF-α(pg/mL) 实验组 97.60 ± 6.29 111.06 ± 5.64 119.60 ± 5.16 111.91 ± 5.79 对照组 103.98 ± 7.84* 120.15 ± 6.42* 131.86 ± 3.74* 138.48 ± 5.34* 与实验组比较,*P < 0.05。 表 4 大鼠支气管组织切片炎症程度对比分析(n)
Table 4. Comparative analysis of inflammation in bronchial tissue sections of rats(n)
组别 n 炎症程度 Z P − + ++ +++ 实验组 32 0 17 11 4 −3.006 0.002* 对照组 32 0 6 14 12 与对照组比较,*P < 0.05。 表 5 大鼠肺泡组织切片炎症程度对比分析(n)
Table 5. Comparative analysis of inflammation in alveolar tissue sections of rats(n)
组别 n 炎症程度 Z P − + ++ +++ 实验组 32 3 19 8 2 −2.493 0.013* 对照组 32 0 13 14 5 与对照组比较,*P < 0.05。 -
[1] Enkhbaatar P, Pruitt BA, Suman O, et al.Pathophysiology, research challenges, and clinical man-agement of smoke inhalation injury[J]. Lancet, 2016, 388(10): 1437-1446. [2] Feng T, Zhou L, Gai S, et al.Acacia catechu (L.f.) Willd and Scutellaria baicalensis Georgi extracts suppress LPS-induced pro-inflammatory responses through NF-кB, MAPK, and PI3K-Akt signaling pathways in alveolar epithelial type Ⅱ cells.[J]Phytother Res.2019, 33(12): 3251-3260. doi: 10.1002/ptr.6499 [3] 贾赤宇, 舒彬. 急性吸入性肺损伤发病机制和临床诊治的相关问题[J]. 中华损伤与修复杂志, 2016, 11(02): 81-83. [4] 钱何布, 赵宏胜. 右美托咪定的抗炎及器官保护作用研究进展[J]. 医学综述, 2015, 21(15): 2706-2709. doi: 10.3969/j.issn.1006-2084.2015.15.007 [5] 李亮, 李军, 米卫东. 右美托咪定抗炎作用研究进展[J]. 北京医学, 2015, 37(4): 369-371. [6] 韩霜, 何锟, 张婧, 张秀果, 李建立, 容俊芳. 雾化吸入布地奈德复合沙丁胺醇对单肺通气致兔肺损伤的预防作用及机制探讨[J]. 山东医药, 2020, 60(15): 47-50. [7] KISS T, SILVA P L, HUHLE R, et al.Comparison of different degrees of variability in tidal volume to prevent deterioration of respiratory system elastance in experimental acute lung inflammation[J]. Br J Anaesth, 2016, 116(5): 708-715. doi: 10.1093/bja/aew093 [8] Miller Andrew C, ElaminElaminM, Suffredini Anthony F. Inhaled anticoagulation regimens for the treatment of smoke inhalation-associated acute lung injury: a systematic review.[J]. Pub-med, 2014, 42(2): 413-419. [9] Dakhama A, Larsen GL, Gelfand EW. Calcitonin gene-related peptide: role in airway homeostasis.[J]. Curr Opin Pharmacol, 2004, 4(3): 215-220. doi: 10.1016/j.coph.2004.01.006 [10] Sekizawa S, Joad JP, Bonham AC. Substance P presynaptically depresses the transmission of sensory input to bronchopulmonary neurons in the guinea pig nucleus tractus solitarii.[J]. Physiol, 2003, 552(2): 547-559. doi: 10.1113/jphysiol.2003.051326 [11] Fontan JJ, Cortright DN, Krause JE, Velloff CR, Karpitskyi VV, Carver Jr TW, et al. Substance P and neurokinin-1 receptor expression by intrinsic airway neurons in the rat.[J]. Physiol Lung Cell Mol Physiol. 2000, 278(2): 344-355. [12] Palmieri TL. Inhalation injury: research progress and needs.[J]. J Burn Care Res, 2007, 28(4): 549-554 doi: 10.1097/BCR.0B013E318093DEF0 [13] You K, Yang HT, Kym D, Yoon J, Haejun Y, Cho YS, et al. Inhalation injury in burn patients: establishing the link between diagnosis and prognosis.[J]. Burns, 2014, 40(8): 1470-1475. doi: 10.1016/j.burns.2014.09.015 [14] Colohan SM. Predicting prognosis in thermal burns with associated inhalational injury: a systematic review of prognostic factors in adult burn victims.[J]. J Burn Care Res, 2010, 31(4): 529-539 doi: 10.1097/BCR.0b013e3181e4d680 [15] Wang W, Zhang J, Lv Y, et al.Epidemiological Investigation of Elderly Patients with Severe Burns at a Major Burn Center in Southwest China.[J]. Med Sci Monit, 2020, 26: e918537-1-e918537-12. [16] Hamilton TJ, Patterson J, Williams RY, et al.Management of Head and Neck Burns-A 15-Year Review.[J]. J Oral Maxillofac Surg, 2018, 76(2): 375-379. doi: 10.1016/j.joms.2017.09.001 [17] Joana A, Flávio R.Dexmedetomidine: current role in anesthesia and intensive care.[J]. Rev Bras Anestesiol, 2012, 62(1): 118-133. doi: 10.1016/S0034-7094(12)70110-1 [18] 雷桂玉. 右美托咪定抗炎和脏器保护研究进展[J]. 中国分子心脏病学杂志, 2017, 17(4): 2193-2196. [19] Son Ga-Yeon, Shin Dong Min, HongJeongHee. Bacterial PAMPs and Allergens Trigger In-crease in[Ca(2+)]i-induced Cytokine Expression in Human PDL Fibroblasts.[J]. 2015, 19(3): 291-297. [20] 吴志林, 褚淑娟, 姚尚龙, 伍静. 不同剂量右美托咪定预处理对大鼠心肌缺血再灌注损伤以及炎症反应的影响[J]. 华中科技大学学报(医学版), 2015, 44(4): 445-447. [21] Taniguchi Takumi, KuritaAkihide, KobayashiKyoko, YamamotoKen, Inaba Hideo. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats.[J]. Journal of anesthesia, 2008, 22(3): 221-228. doi: 10.1007/s00540-008-0611-9 [22] Lili Jiang, Li Li, JinmeiShen, et al. Effect of dexmedetomidine on lung is chemia reperfusion injury. 2014, 9(2): 419-426. [23] Geng Y, Li R, He SX, et al.Dexmedetomidine Attenuates Acute Lung Injury Induced by Heatstroke and Improve Outcome.[J]. Shock, 2019, 52(5): 532-539 doi: 10.1097/SHK.0000000000001289 [24] Liu J, Huang X, Hu S, et al.Dexmedetomidine attenuates lipopolysaccharide induced acute lung injury in rats by inhibition of caveolin-1 downstream signaling.[J]. Biomed Pharmacother, 2019, 118(1): 109-314. [25] Liliensiek B, Weigand MA, Bierhaus A, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response[J]. The Journal of clinical investigation, 2004, 113(2): 1641-1650. [26] Zhang Y, Tan X, Xue, L. The alpha2-adrenoreceptor agonist dexmedetomidine protects against lipopolysaccharide-induced apoptosis via inhibition of gap junctions in lung fibroblasts[J]. Biochem Biophys Res Commun, 2018, 495(1): 92-97. doi: 10.1016/j.bbrc.2017.10.162 期刊类型引用(2)
1. 康曦,李琴,刘方. 循环血肿瘤细胞检测在根治性膀胱全切术后评估中的临床研究. 中国当代医药. 2021(10): 123-126+241 .  百度学术
百度学术2. 徐兰锋,朱丹,袁潮. 生存蛋白在膀胱癌患者尿液脱落细胞中的表达及临床相关性研究. 中国实验诊断学. 2020(02): 316-319 .  百度学术
百度学术其他类型引用(0)
-






 下载:
下载: